The world has enough natural gas to power it for many centuries. The idea of clean, abundant natural gas is terrifying to people who have invested heavily in nearly worthless technologies like solar and wind powered electricity. This has led to idiotic claims that methane is 20X worse than CO2 and will cause mankind to go extinct.
The truth is that methane has almost no effect on Earth’s radiative balance. It has only a couple of narrow absorption bands, both of which overlap with much more abundant H2O. The atmospheric mole fraction of CH4 is less than 0.000002

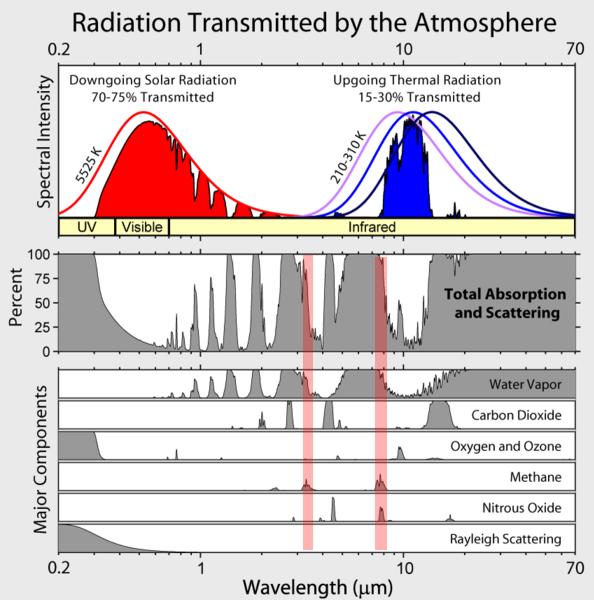

I love the way those “210 -310K” lines overlap and have equal areas – which is contrary to the laws of physics.
Umm – of course they’re going to overlap, when the temperatures are just slightly different. At what temperature change shouldn’t they overlap? 10C? 1C? 0.1C? 0.01C…
And have you ever heard of a normalized plot?
For 210K the area under the curve (the intensity w/m2) should be 110, for 260K, 260 and for 310K, 523.
All three curves should start off together on the left, but the 260K curve should rise higher and go farther than the 210K curve. The 310K should rise higher and go farther still.
I’ve heard of a normalised plot, but I don’t know what it means.
Averaged! Or better yet, WAG. It could even refer to the “Mean” of the Highest and lowest as is done when referring to temperature. It is just a number that has no value in the real world except for those that belong to the Chicken Little Brigade. The same ones that claim H2O has a short residence time in the atmosphere. While that may well be true for any individual molecule of H2O vapor, it ignores the constant vaporization of H2O that maintains a very high level in the atmosphere at all times. Relative to the other so called GHGs.
When separate values are Normalized they remove whatever value the individual numbers may have had and reduce the results to trash or fairy tales.
@ Miked1947
So it soundslike “normalised” means “so horribly distorted as to create a completely misleading impression.” Is that it?