Trenberth claims that the missing heat has sunk to the bottom of the ocean. This defies basic physics and common sense.
Any place where surface water is more than 5C, the density decreases as the temperature warms. Heating the surface of the ocean will not make the water sink.
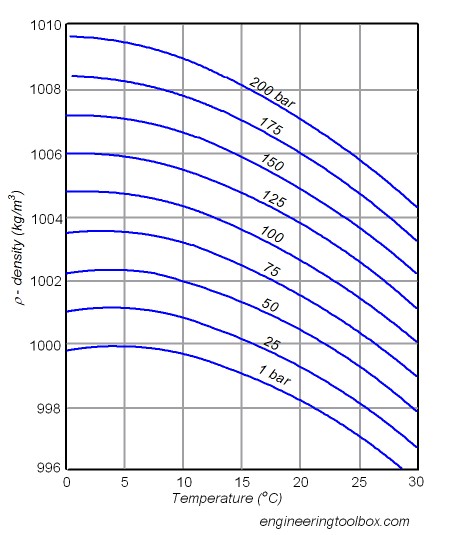
The only place where warm water sinks below cold water is at the surface near the poles. At one bar, 5C water is more dense than 0C water. However at 100 bars, the density of 0C water is highest.


Reblogged this on Climate Ponderings.
Lakes in the north do not freeze with any more that a few feet of ice because to freeze the water must expand and the pressure is too great. Water is most dense at about 4 deg C. To warm or cool from that temp it must expand. Every 32 ft greater depth is another atmosphere of pressure.
At the depths Trenberth has suggested heat is sequestered, even a small fraction of a degree temp change is bizarre at that pressure.
Also, he would have us believe that the heat was magically transported down to those depths since the Argo system did not record any movement of heat downward. If by some magical means it was down there, there should be convective forces causing the heat to rise. His sophisticated explanation seems like a desperate attempt to explain an inconvenient truth.
‘Scientists cannot explain the tragedy of not knowing where the missing heat is, and will not except the idea that the missing heat never was’
DarrylB.
You are 100% correct. It`s all to do with atomic structure.
Yes water at 4 deg C is denser,that is why fish can survive in frozen lakes..
Is this where all the missing heat goes? (Major sarc,Ha Ha)
This is school boy science.Not Algore science.
Terry—yeah and the beautiful thing is that the unique angular structure of water, unlike CO2 for instance which is a straight molecule means that lakes and oceans do not solidify under pressure. If water did contract when it turned to a solid, the lakes and oceans would all be frozen under the pressure and we would not have life on earth as we know it.
Also, perhaps the terms Al Gore and Science should not be used in the same sentence!
…every 34 feet.
Harry–please check it out. The increase in pressure would be another atmosphere (about 14.7 pounds per inch squared) for every 33 feet increase in depth. However, sea water is denser so it would be another atmosphere of pressure for every 32 feet increase in depth. The important thing upon which we agree is that the pressure is about a ton and a half of force on one square inch at a depth of 2,000 m.
So to find the missing heat all they have to do is accurately measure the temperature of the water at depth. Any increase from the max density temperature and viola!there it is. What could be easier?
There is no way for heat to get to the depths suggested by Trenberth other than conduction. There are no transport mechanisms to get the heat to those depths. And if the heat already exists in the oceans, we should be seeing it in a sea level rise that reflects the theory. That rise would need to be exponential to support the CAGW claims. Linear or Logarithmic won’t meet the requirements for “catastrophic”.
Within the cult of Gaia, the laws of physics are suspended by the forces of Petulant desire. Think of it as the ‘Ebonics’ of the Physical Sciences. Understanding of this new Science is best achieved with psychotropic agents like LSD, taken liberally thru-out collegiate years. A doctorate level of understanding is achieved when you can see the gumdrops and purple eyeballs floating in the sky. This also qualifies you for employment on the Obama surveillance squad.
There is a bigger issue beyond the OHC and that is the precision of the measurement. If I’m not mistaken the overall warming in terms of temperature change of the oceans is on the order of 0.1C and that is considering that very sparse measurements prior to the Argo Buoys. And even the over all measurement precision is also on the order of a 0.1 C for Argo so the reality of heat being lost in the ocean is that it’s so small it buried in the noise.
You see significant up and down movements of heat by just keeping an eye on water at depth in ENSO regions so I don’t understand what Steve is claiming. Just look at the heat profile graphs…
That is being driven by wind powered convection, not density differences.
Re: “The only place where warm water sinks below cold water is at the surface near the poles. At one bar, 5C water is more dense than 0C water. However at 100 bars, the density of 0C water is highest.”
Interesting, I did not know that! But your graphs are for fresh water. Is the statement true for seawater, too?
(A nit: of course, you really mean to say liquid water. if the “cold water” in question happens to be frozen (solid), then warm water will certainly sink beneath it.)
That basically follows the same curve. Ice at 0C is less dense than 1C water, as shown in the diagram.
Hi Steven,
I took the liberty of posting your graph-plus my commentary–at the world’s smallest social networking site, free-associationdotnet.
LINK http://tinyurl.com/mewspy6
Of course, I gave you credit for the graph. When I have the time, I’d like to a longer article on the subject at HubPages. Anything worth doing is worth overdoing. Do I have permission to use your graph there?
Nice looking site.
My understanding is that sea water doesn’t exhibit quite the same characteristics, at least near 0°C. The curves in your diagram above shift up and to the left, such that the maximum disappears for the purposes of our discussion. The 200 bar curve is adequate as an exampe of the shape. Sea water also doesn’t freeze at 0°C, rather it’s a little lower, at about -2°C.
In essence, warm water never sinks in the ocean, so it’s a bit simpler than pure water.
When the surface waters are cooled dramatically near the poles due to convection (freezing winds) and radiation, they become colder and more dense than the shielded waters below, and so they sink. The water temperature at this point is in the range 0°C to -1.9°C. This process contributes to the deep ocean currents. It usually only happens in bulk at the poles, because it’s the only place where such intense surface cooling takes place relative to the underlying layers.
The only way warmer sea water can sink is if its salinity increases a lot, but this is greatly swamped by temperature, which is the main driver of water density.