http://math.nyu.edu/~gladish/teaching/eao/water-phase-diagram.jpg
A person inexperienced with interpreting phase diagrams would conclude that no water vapour (gas phase) can exist below the boiling point.
Consider the red circle. Temperature is 40C and one atmosphere pressure – a typical day in the Amazon jungle. The phase diagram shows no gas phase, yet we know the humidity in the Amazon jungle is very high. Obviously, the phase diagram does not represent the real world.
Phase diagrams represent perfect equilibrium conditions in a closed system, which never exist on earth. A skillful scientist understands this, yet uses the important information contained in the phase diagram to her/his benefit.
In the Arctic, when temperatures are close to 0C – water exists in all three phases solid/liquid/gas. We are all familiar with this. We don’t have to drop the atmospheric pressure to the boiling point of water at 0C (0.00603 atmosphere) to have water vapour in the air.

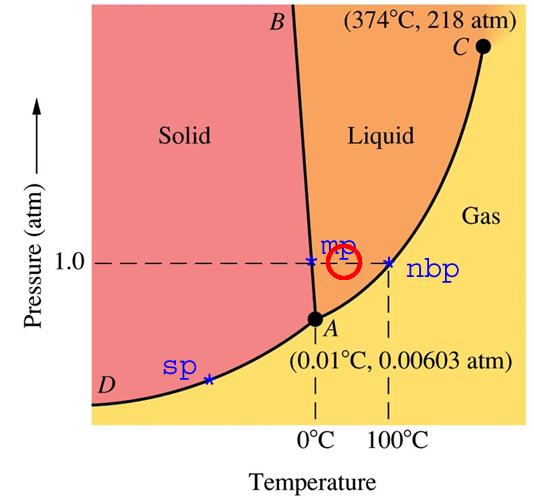

Steve, if you draw a vertical line down from point mp to the curve AC, the point where it intersects will tell you the equilibrium partial pressure of water vapor at that temperature. That is how to use a phase diagram.
So in fact this diagram is very useful for describing real world conditions.
This will be my one post on your blog.
Bye.
[jeez – Water exists in all three phases in the Arctic. That is the definition of a triple point. Good luck to you.]
Steve, jeez is correct! Why did you diss him?
[Ed – I appreciate your input. If you disagree with this post, let’s talk science. Thanks!
jeez said this will be his only post on this blog, so I wished him good luck]
Hi Steve,
I’m working on an e-mail to CTM at WUWT to see if I can get the final conclusion of the Sea Ice News #20 changed (this may take a few days, as I’m really busy and want lots of links/references). I just don’t like seeing WUWT shut off discussion with the wrong conclusion stated at the end. I don’t agree with how your argument was presented, but you were essentially correct (at least you were much more correct than the people disagreeing with you). I suggest you point people who don’t understand to:
http://en.wikipedia.org/wiki/Triple_point
Here, it says:
However, people don’t seem to get the concept of vapor pressure and partial pressure, as they don’t get that the partial pressure has to be less than or equal to the vapor pressure (almost certainly exactly equal in the Arctic).
I also think that the phase diagrams just confuse people because they are only strictly valid for pure water. Here, we have the effect of an (effectively) inert gas adding pressure to the system, which makes it less straightforward.
-Scott
[Scott thanks. I think it is pretty clear that all three phases exist at the same place and time at 1 bar in the real world. Some people took just enough chemistry to be dangerous]
I’ve never understood why people think that there could be liquid water on Mars. There pressure on Mars is 30 Pascals or .0003 atmospheres. At this pressure, it is impossible at any temperature for water to be in the liquid phase.