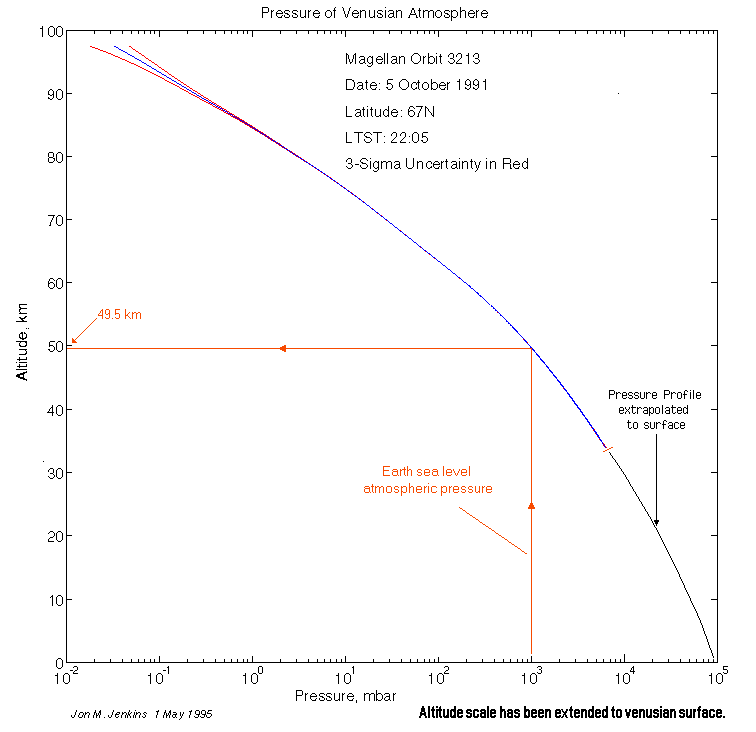
At 50km, the atmospheric pressure is about the same as Earth.
http://www.datasync.com/~rsf1/vel/1918vpt.htm
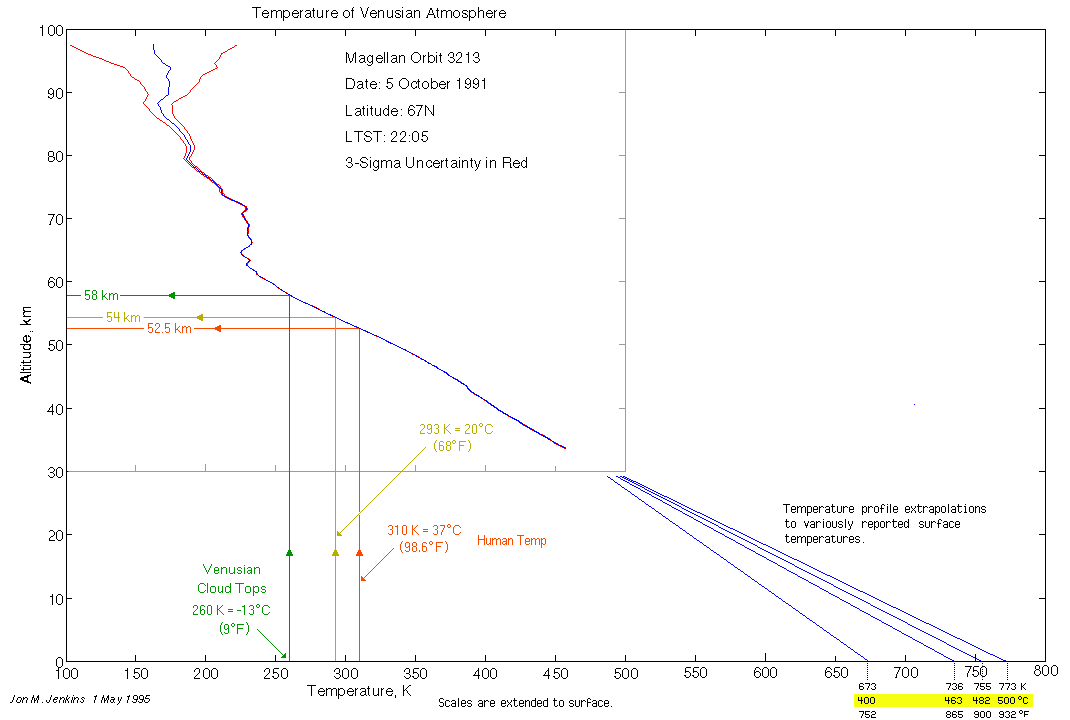
At 50 km, the temperature is also about the same as Earth.
At the same pressure, the temperature is similar- yet the atmospheric chemistry is very different.
The Ideal Gas Law does not have a term for CO2.



But there is a molecular weight term…you can write p = ?R’T/?, where R’ is the gas constant and ? is the mean molecular weight. This form is used by astronomers a lot and helps explain why stars evolve as they do (because hydrogen is converted to helium in the core), but atmospheric scientists typically call R’/? its own constant (call it R) because they don’t need to deal with a changing ?, as that is dominated by the nitrogen and oxygen content.
The whole point of the Ideal Gas Law is that it represents an “ideal” gas – and doesn’t worry about the minor effects you describe.
The ideal gas laws are the equation of state for Earth’s atmosphere but not for Venus, the equation of state appropriate there does have a term for CO2! They aren’t minor terms either, compressibility is around 0.5.
I see. Venus atmosphere behaves the same as Earth because it is different.
stevengoddard says:
April 17, 2011 at 2:34 pm
I see. Venus atmosphere behaves the same as Earth because it is different.
Well Earth’s troposphere obeys P=dRT whereas Venus’s troposphere does not, what makes you think they behave the same?
Because the temperature in Venus’ atmosphere is the the same as Earth’s at the same pressure. That was the point of the article.
Chris, wtf? Do you think the readers here believe stuff just because Steve says so? You biases and prejudices are transparent.
Your first comment was almost there….. and then you go tangent.
The ideal gas law is an equation. Stuff on one side and stuff on the other. Applying basic math principles one can plainly see pressure isn’t the only factor in determining a temperature. I don’t believe Steve is arguing that. However, one can also plainly see that given Venus’ pressure, it is a significant factor in determining Venus’ temps.
Now, if one wants to argue, as Phil did, that basic physical laws on earth don’t apply to Venus, then we have a different discussion.
Steve is also correct, in that you’re not stating a case very well. Slow it down and lose the condescension, people may be more receptive to your arguments.
Damn replied to the wrong person with the wrong comment.
Phil, “The ideal gas laws are the equation of state for Earth’s atmosphere but not for Venus…”
Beautiful Phil, I guess we can now dispense with the comparisons of Earth to Venus regarding gases and climate. I second your motion! We’ll get to a consensus yet!!
If that were true it would only the same at a single point since the planets have different lapse rates Venus, 10.5K/km vs 9.76K/km for Earth. As I pointed out the behavior of density as a function of Temperature and Pressure is dramatically different in the two atmospheres. However your graphs don’t even show what you claim, 340 K at 1 bar is rather hotter than anywhere I can think of on Earth.
Venus is a little warmer at the same pressure. The vast majority of the temperature difference on the surface is obviously due to pressure, and I am astonished that people continue to argue about it. This is really fundamental stuff, and Sagan’s legacy of cluelessness is not an excuse.
Actually, people are still astonished that you seem to think all of planetary climate is reducible to a gas law (which you don’t understand) with absolutely no consideration for quantum mechanics & radiative transfer, conservation of energy, or direct observation for that matter. It has nothing to do with Sagan, but your continued misunderstanding and misdirection leads me to question why anyone actually continues to take you seriously? I also think your blog would be an interesting case study for a psychologist.
Actually, you are doing everything you can to avoid facing the obvious fact that the high surface temperatures on Venus are due to the high atmospheric pressure. If Earth’s atmosphere were as thick as Venus, temperatures would be about the same.
This is very basic physics and the fact that you continue to obfuscate does your side no credit. Kind of pathetic really.
Chris, wtf? Do you think the readers here believe stuff just because Steve says so? You biases and prejudices are transparent.
Your first comment was almost there….. and then you go tangent.
The ideal gas law is an equation. Stuff on one side and stuff on the other. Applying basic math principles one can plainly see pressure isn’t the only factor in determining a temperature. I don’t believe Steve is arguing that. However, one can also plainly see that given Venus’ pressure, it is a significant factor in determining Venus’ temps.
Now, if one wants to argue, as Phil did, that basic physical laws on earth don’t apply to Venus, then we have a different discussion.
Steve is also correct, in that you’re not stating a case very well. Slow it down and lose the condescension, people may be more receptive to your arguments.
No, it isn’t. Read a textbook. You can switch your logic around as well and say that the high pressure is due to the high temperature based on pV=nRT, but that would just be the same misapplication of atmospheric science.
If you were right, you could revolutionize the field of planetary climate, atmospheric science, and physics– but you know you are not, which is why you continue to blog about it and confuse your readers who can’t really tell the difference, instead of publishing about it. It’s the same tactics you pull at WUWT and on other subjects, like on sea ice.
Do you doubt that the temperature on Earth would be similar to Venus if the atmosphere were as thick?
Just answer the question yes or no – and quit trying to obfuscate.
If you kept the energy balance parameters like albedo and IR absorption the same, no it wouldn’t. You can generate some additional greenhouse effect by adding a 90 bar N2 atmosphere, but your argument is simply that the increased pressure would cause a new, much higher surface temperature. That’s just wrong. Sorry.
Well that shows your ignorance. Why is it 40F hotter at the bottom of the Grand Canyon than at the top? I can assure you that the atmospheric chemistry is identical in both locations.
Do a Google search for dry adiabatic lapse rate.
Steve– I can assure you I understand the lapse rate very well, as do thousands of planetary and atmospheric scientists. Of course the lapse rate sets the temperature gradient, but you cannot isolate the temperature at any single point with no information other than the lapse rate. You need to know something about the sun, the albedo, the atmosphere, etc. You minus well have your readers believe that the temperature is independent of all energy terms and only depends on pressure.
My recommendation: Do a google search for the Dunning–Kruger effect
Einstein said :
“It can scarcely be denied that the supreme goal of all theory is to make the irreducible basic elements as simple and as few as possible without having to surrender the adequate representation of a single datum of experience”
Venus troposphere is about 60km thick. Earth’s lapse rate is1C/100 metres, that gives us a delta of 600K. This explanation is completely adequate and it is beyond my comprehension why you insist on looking for unnecessary complexity.
But the thickness of the troposphere is not independent of the GHG content, another thing you don’t understand.
Please spare us the bullshit. If you dug a hole 50 km deep on Earth, it would have 60 km of troposphere above it. The composition would be the same and the temperature would be very hot. Like Venus.
You’re always welcome to dig the hole, go down, and verify for us 🙂
In any case, for readers actually interested in the subject, the tropopause height depends largely on the lapse rate and, in the case of Venus, the optical thickness. Venus is almost in an ‘all-troposphere’ limit, with very cold upper atmospheric levels due to IR cooling by CO2 (much like how increasing CO2 cools the stratosphere).
I’m done conversing with Steve, who clearly knows nothing and doesn’t want to. I’ll monitor the posts for comments worth responding to, but I’ll just suggest that people actually interested pick up some books on the physics of planetary climate. Ray Pierrehumbert’s is very good, Houghton’s is good, Hartmann is decent. You’ll find all of them recognize the important of the greenhouse and planetary energy budget (oh, and they all talk about the ideal gas law too)
Really pathetic.
I’ll also leave a request, to satisfy myself and your readers.
As I am sure you aware, there is an enormous literature on Venus. I would like you to point just one document in the refereed literature that attributes the hot surface temperatures of Venus to the high pressure, and not the greenhouse effect. Just one. If you can’t, please then explain how so many planetary scientists skipped out on undergraduate physics when they talked about the ideal gas law. I’d like to hear this. And no “hole-in-the-ground” obfuscations, just one resource.
Just goes to show once again how incredibly lame the state of climate science is.
I mean, you’re also allowed to consult physics journals, astronomy and astrophysics journals, whatever journals actually talk about Venus…are they all lame too?
(I am “omnologos” for those unfamiliar with my net presence)
The parallels with the Ediacara fauna are striking. There too, experts spent decades “demonstrating” that what looked like fossils, ain’t so. At least 4 people threw away their scientific reputation in the futile attempt of showing the scientific community what any schoolchild can see very clearly, i.e. that what looked like fossiles, it was fossils indeed.
Likewise, with Earth’s and Venus’ atmosphere behaving similarly in similar conditions, there we go with megabytes of expert opinions saying, it ain’t so.
And BTW for the Coloses of this world…yes, people did try to get their Ediacara papers published in peer-reviewed publications. Guess what? “Nature” deemed their work not good enough for publication.
Then a big gun of paleontology got convinced (bless him!) and guess again what? “Nature” deemed his work good enough for publication. Overnight, everybody got convinced that the Ediacarans were animals indeed.
lol, oh Omno, that’s just history stuff. No sense in learning from that. That just doesn’t apply here. We’re way smarter now! No way we can be wrong about simple stuff like the atmospheric science of Venus. You see, once we figured out all that we needed to know about earth’s climate, then Venus was a snap! It’s like CO2 causes runaway GH effect. We can see it here on earth in a milder form……
http://suyts.files.wordpress.com/2011/04/image_thumb.png?w=657&h=421
Ok, so in a paradoxical form. But that’s no big deal, because we learned that some laws apply to Venus and others don’t.
suyts says:
April 18, 2011 at 4:09 am
Now, if one wants to argue, as Phil did, that basic physical laws on earth don’t apply to Venus, then we have a different discussion.
The ideal gas law isn’t applicable to the Venusian atmosphere (it isn’t applicable on Earth either to a container of 92 bar gas at 450ºC, mainly CO2). For those conditions you’d need the van der Waals equation or the Redlich-Kwong equation as your equation of state as there are considerable deviations from the ideal gas law.
The ideal gas law is applicable to earth inthat the critical temperature of nitrogen is so far away from that experienced on earth we can use it and be within 1% error. One per cent is close enough according to thermodynamics book.
That wasn’t at issue, use it on Venus and you’d be out by as much as 50%.
The atmospheric profile of Venus shows about 20-30C difference from earth at the same pressure. That is nowhere near 50%.
You mean, like yourself?
http://stevengoddard.wordpress.com/2011/04/18/the-lapse-rate-is-independent-of-greenhouse-gas-content/
Phil. says:
April 18, 2011 at 3:51 pm
That wasn’t at issue, use it on Venus and you’d be out by as much as 50%.
stevengoddard says:
April 18, 2011 at 3:53 pm
The atmospheric profile of Venus shows about 20-30C difference from earth at the same pressure. That is nowhere near 50%.
Trouble reading English, note “as much as”? There is probably a point where the curves for the two planets’ atmospheres intersect that doesn’t mean that an ideal gas equation of state is appropriate for the Venusian troposphere, it isn’t.