Yesterday we looked at the difference between the freezing point and dew point (or frost point) of water. Please read that article first.
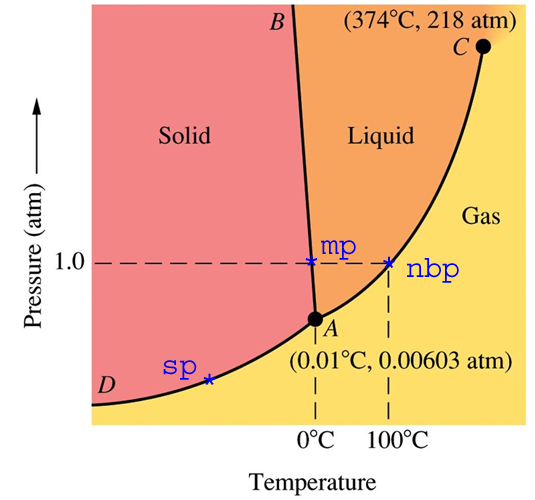
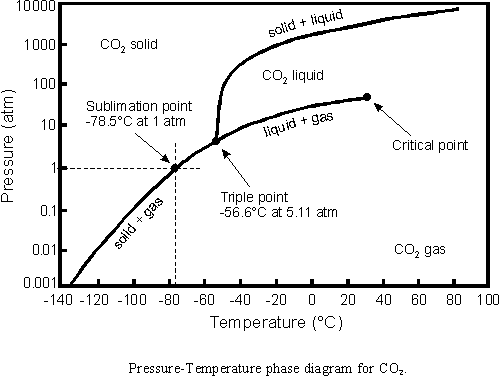
The CO2 phase diagram is not terribly different.
http://www.standnes.no/chemix/english/phase-diagram-co2.htm
The freezing point of CO2 at one atmosphere is -78.5ºC. Temperatures in Antarctica occasionally get colder than that, so dry ice molecules do freeze directly out of the air.
However, they don’t accumulate because the number of subliming molecules is identical to the number of freezing molecules. This is exactly the same principle we discussed for water. There is very little CO2 in the atmosphere. Concentration is less than 0.0004, so not many molecules are available to be frozen. In order to get below the frost point (where CO2 ice starts to accumulate) temperatures would have to be much colder.
The freezing point is fixed at a given pressure. The partial pressure of CO2 does not change the freezing point, but it does change the frost point.


Steve,
I think you’re still getting confused by phase diagrams. Just because you cross a phase line in a phase diagram, doesn’t mean instant change to another phase — i.e. your inference that CO2 will ‘freeze directly out of the air.’ Look at the water phase diagram. Does water freeze directly out of the air anytime the temperature drops below 0ºC? Or does water condense directly out of the air any time the temperature drops below 100ºC. It is clear that it doesnt.
Here’s a good write up on CO2, freezing and Antarctica: http://www.newton.dep.anl.gov/askasci/env99/env188.htm
Matt,
Please read both articles more carefully. Especially the first one.
Ice will not accumulate until it gets below the frost point, which is normally lower than the freezing point.
Does an individual molecule have any knowledge of partial pressure? Of course not. Partial pressure is a statistical measure based on large numbers of molecules.
Accumulation of ice below the freezing point is determined by how many molecules are freezing, vs. how many are sublimating.
Does an individual molecule have knowledge of temperature? Of course not. Temperature is a statistical measure based on large numbers of molecules.
Actually, yes it does. The partial pressure measures how much CO2 is present in a given volume of space – in physical terms, that tells you how often one CO2 molecule is likely to collide with another CO2 molecule and potentially stick to it (i.e. crystallise out as a solid).
Total pressure, in contrast, measures how much “everything” is present in a given volume – in physical terms, how often one CO2 molecule is likely to collide with *any other molecule*. That isn’t relevant to the dynamics of freezing / melting / sublimation – if a CO2 molecule bumps into an N2 molecule or an O2 molecule, they’re not going to stick together and crystallise out as a solid.
Think of it as analogous to dissolved material. If you have a weak salt solution (low partial pressure) it’s not going to crystallise out. If you add more salt or remove some of the water, the concentration will go up (higher partial pressure) and it will start to crystallise out. You can increase the *total* concentration of dissolved solids (analogous to the total pressure) by adding sugar – that won’t magically make the salt crystallise out.
(missing sentence from my first paragraph)
An individual CO2 molecule “sees” an increase in the partial pressure of CO2 as an increase in the number of other CO2 molecules it bumps into as it moves around – so yes, it does in effect have “knowledge” of the partial pressure.
Frost versus dew points (a few opening paragraphs will suffice):
http://www.theweatherprediction.com/habyhints/347/
http://www.shorstmeyer.com/wxfaqs/frost/frost.html
http://www.britannica.com/EBchecked/topic/220910/frost-point
Regards
Interesting views on the subject.
From probabilistic point of view there may exist H2O molecules in freezing state at 100 C deg!
Imagine one of them being hit from 6 perpendicular directions by her sisters and thus stopping the “chosen one” what is tantamount to depriving her of energy and making her to slip into freezing zone (from energy point of view). 😉
But, as other “sister” H2Os are very jealous of the privileged state they attacked the Princess in a blink of an eye pushing her into higher energy state corresponding to 100 C deg temperature.
So, may the water vapor freeze directly out of the air or not? 😉
Regards
Steven, you are confused about phase diagrams. In a volume of mixed gases, the pressure scale on the ordinate of the diagram is ALWAYS the partial pressure of the substance in question and NEVER the total atmospheric pressure.
Not to mention that phase change, like condensing and freezing, are bulk properties of masses of molecules of a substance. Individual molecules cannot condense or freeze.
(Not to mention that water doesn’t have just one triple point, it has numerous triple points.)
http://www1.lsbu.ac.uk/water/phase.html
Brego,
Phase diagrams assume a closed system, with no other species. Nevertheless, we can infer useful information from them in the real world. At sea level, water boils at 100C, because the atmospheric pressure is 1 atmosphere. As seen in the phase diagram.
Up here in Colorado, water boils at a lower temperature, because the atmospheric pressure is lower.
Regardless, we always have evaporation and condensation occurring – no matter what the temperature is.
An ice crystal has to start with an individual molecule, normally nucleating on an impurity.
So the frost point for CO2 is about -140 C?
I’m not sure what it is exactly, but it gets a little higher every year ;^)
Nice one, Steven!
The freezing point of CO2 at one atmosphere is -78.5ºC. Temperatures in Antarctica occasionally get colder than that, so dry ice molecules do freeze directly out of the air.
Versus:
The freezing temperature of pure carbon dioxide at one atmosphere of pressure
is -78.5 C (-109.3 F). In the Earth’s atmosphere, at sea level, carbon dioxide
constitutes only about 0.0004 atmosphere of partial pressure. Partial pressure
refers to the amount of force (which is basically equivalent to weight in the
Earth’s atmosphere) that the gas exerts in air. At such a low partial pressure,
a temperature of less than about -140 C is needed for carbon dioxide gas to be
converted to solid carbon dioxide. At such low temperatures and one atmosphere
pressure, carbon dioxide can not exist as a liquid, only as a solid. Since the
lowest recorded temperature on Earth is -89.2 C at Vostok Station, Antarctica, it
can be safely said that carbon dioxide gas has not frozen out of the air anywhere
on Earth in recorded history.
Furthermore, since Vostolk is not at sea level, the atmospheric pressure there is
less than one atmosphere, resulting in less dense air, a lower partial pressure of
carbon dioxide than at sea level, and thus an even lower temperature being required
to convert carbon dioxide gas to a solid.
The temperature being at “freezing”, the temperature usually defined as resulting in
a phase change from pure liquid to pure solid at one atmosphere of atmospheric
pressure, does not imply that the solid phase of carbon dioxide will result at that
temperature in the atmosphere. The partial pressure of the gas must be high enough
for “saturation” of the air to occur (where the air can no longer hold all of the
element as gas without some changing to the liquid or solid phase). At Vostok,
Antarctica, the carbon dioxide content of the air is not high enough and the air
temperature is not low enough for carbon dioxide gas to reach the saturation partial
pressure (referred to as saturation vapor pressure for water); therefore carbon
dioxide gas will not freeze out of the air.
(…)
However, they don’t accumulate because the number of subliming molecules is identical to the number of freezing molecules.
(…)
In order to get below the frost point (where CO2 ice starts to accumulate) temperatures would have to be much colder.
In simple words – the molecules are in balance without any accumulation at present (real) conditions and would be in balance until reaching “below the frost point”.
The first part recalls me Feynman diagrams and spontaneous creation of particles “out from the space”. The heavier the particles the shorter the time they can exists in. So in turn make them illusive to our senses until some of them decay into energy photons.
The same way we are not able to “see” dry ice in Antarctica until the physical conditions of the air are more favorable to the solid CO2 (below the frost point).
This “freeze out from the air” looks in the post like “freeze out from the hat”. 😉
What did I miss? (I’m trying to find out the crux of the problem here…)
Regards
Matt says:
September 5, 2010 at 7:37 pm
I can take a fair amount of credit for that – before summer last year it said CO₂ frost could form at Vostok. I contacted Argonne and the author and quickly got back a nice note from David Cook, acknowledging I was right and that the web page would be fixed.
http://wattsupwiththat.com/2009/06/09/co2-condensation-in-antarctica-at-113f/#comment-143154
Ric,
Right, but I never said it would accumulate. Obviously there isn’t any dry ice at the South Pole. That is the whole point of this article – pointing out the difference between the freezing point and the dew point.
The purpose of the WUWT article was to remind everyone (in a tongue-in-cheek fashion) that it is really cold in Antarctica. In fact cold enough to cause Dr. Hansen’s “primary greenhouse gas” to freeze.
I didn’t write the Argonne article.
> I didn’t write the Argonne article.
That’s why I sent the correction to the author, Dr. Cook.
Brego says:
September 5, 2010 at 10:11 pm
> Steven, you are confused about phase diagrams. In a volume of mixed gases, the pressure scale on the ordinate of the diagram is ALWAYS the partial pressure of the substance in question and NEVER the total atmospheric pressure.
Between the discussion here and last year on WUWT, I decided I didn’t know as much about phase diagrams as I thought, and became convinced that no one else did either.
My current thinking is that the phase diagrams one typically sees provide information about the pure chemical in question. More about that below.
> Not to mention that phase change, like condensing and freezing, are bulk properties of masses of molecules of a substance. Individual molecules cannot condense or freeze.
I agree we should be talking about bulk properties, but events happen one molecule at a time. I would be happier if Steve included a block of dry ice in his system. The system would not be stable – the dry ice would be sublimating.
> (Not to mention that water doesn’t have just one triple point, it has numerous triple points.)
> http://www1.lsbu.ac.uk/water/phase.html
Come, come. If you’re going to point out we should be talking about bulk properties, I think you should also limit discussion to situations that occur in contact with the atmosphere. Water vapor at Gigapascal pressures adds no clarity and is not relevant to the discussion.
For your water phase diagram, I’d discard most of it, leaving just the just the temperature range from about 170-400k, and pressure from 200 Pa to 200 KPa. That covers the high atmosphere to ground level and temperatures from colder than occurs naturally to above boiling.
A lot of this discussion started as a look at events in the Arctic, where the freezing point of sea water is not 0°C. Therefore, that discussion should have involved a phase diagram of sea water, not pure water.
I think a major source of confusion, but in practice not an issue as far as some of the curves go for typical chemicals is exactly what the pressure axis means. For pure chemicals, it’s not an issue, for our Arctic phase diagram, then the pressure is pretty much within 5% of normal atmospheric pressure, which is about 100 Kpa. Let’s use atm as above, not as with the phase diagram you referenced.
A relevant phase diagram will refer to a system of both seawater and air – the extra pressure from the air will have a very small effect on the freezing point, so instead of plotting that, plotting the partial pressure of water vapor is the item of interest.
This makes for a phase diagram that reads very differently. The pure water phase diagram says there is no water vapor at a 1 atm pressure until the water reaches the boiling point. That’s not a problem – imagine a seal bag of pure water at room temperature and pressure. No vapor. Freeze it – no vapor. No surprise. The pressure of the bag forces the stray energetic molecule back into the colder bulk. Open the bag, let in some air, reseal it, and now the system is no longer stable – a few molecules can break away and collisions with air molecules may or may not force them back into the liquid or solid. Eventually things will stablize with the water vapor being at the partial pressure given by the phase diagram. (I suspect the sea water phase diagram will have a measureably lower partial pressure given the hygroscopic nature of sea salts.)
Przemys?aw Pawe?czyk says:
September 5, 2010 at 8:52 pm
> So, may the water vapor freeze directly out of the air or not? 😉
Sure. If it didn’t, I think frost and snow crystals would look very different.
My mental model is that water vapor molecules collide with molecules in an ice crystal and bind to electrostatically attractive sites. The vapor’s kinetic energy is transferred to several water molecules in the crystal lattice. The physics is not completely understood.
As a matter fo fact, simple air temperature has a huge impact on how ice crystals form. A very useful graph is at http://www.its.caltech.edu/~atomic/snowcrystals/primer/primer.htm – note the link to http://www.its.caltech.edu/~atomic/snowcrystals/primer/xmorphologydiagram.jpg
This is a thermodynamics discussion- not kinetics. At a certain temperature, it will be thermodynamically encouraged for a phase to change. Actually for water to start to freeze stably, it will first need to nucleate (form a an ice seed of a certain size) which depending on the conditions can involve supercooling to -50C in the case of a large pool of pure water. Then the nucleates start to grow and the temperature quickly rises to 0 C. The value of silver Iodide to reduce the supercool needed.