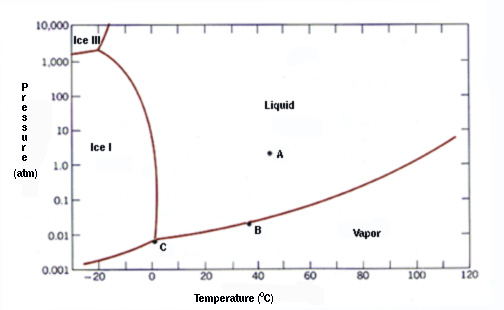
At 0C, a body of fresh water will exist at equilibrium in the three phases solid-liquid-gas, with a vapor pressure of 1/100th of an atmosphere. That is exactly what we measure on Earth. Anyone who has ever had a drink with ice in it, is aware of the fact that the three phases can exist together. Water, ice and vapour condensing on the glass.
If you are above 0C, all the ice will eventually melt. If you are below 0C, all the liquid water will eventually freeze. Vapour will be present at any temperature above absolute zero. There is only one temperature where you can have all three in equilibrium.
Other gases in the atmosphere increase the total pressure, causing the freezing point to depress by a tiny fraction of a degree. The phase diagram is not an exactly 100% perfect representation of the system because of the presence of other gases – but it is close enough.
Why do dim-witted alarmists keep bringing this up?


They are fascinated by fish and prefer “Red Herrings”
http://www.lakemichiganangler.com/tips/bait_salt.htm
Of course we should consider the “Straw Man”
http://www.youtube.com/watch?v=wOKK8mAkiUI
Steve – you left ice-IX off the phase diagram, which is neglectful given how much hot snow fell on the northern hemisphere last winter.
I predict more global warming induced ice-IX snow this winter too.
No! Look, anywhere along the liquid/vapor phase change line, the point is not that vapor and liquid coexist, but rather that they are at a key point where just a small increase in temperature will cause the liquid portion to boil, or a small decrease in temperature will cause the saturated atmosphere to condense. At 1 atmosphere, this key point is at 100 degrees C – eg, boiling. Yes, at 20 degrees C and 1 atmosphere, there is some water in the gas phase based on vapor pressure – this is _not_ the same as boiling, and therefore the water is _not_ on the liquid/vapor phase change line. In the same way, the triple point can only occur when the liquid is simultaneously at a temperature where it is boiling, freezing, and condensing. That just does not happen for water at 1 atmosphere of pressure. This is nothing to do with alarmism – this is just standard high school chemistry. (and, FYI, I have a graduate degree in chemistry)
Do you really believe what you say, or are you just trolling? It seems hard to believe the former, and yet…
Physics – physics maybe?
M, I read your screed three times. I’m a rather intelligent person, but for the life of me I do not know what the f*ck you are trying to say.
If you’re saying “the earth is warming, we’re all gonna die,” I’m sorry to say you’ve failed to convince me.