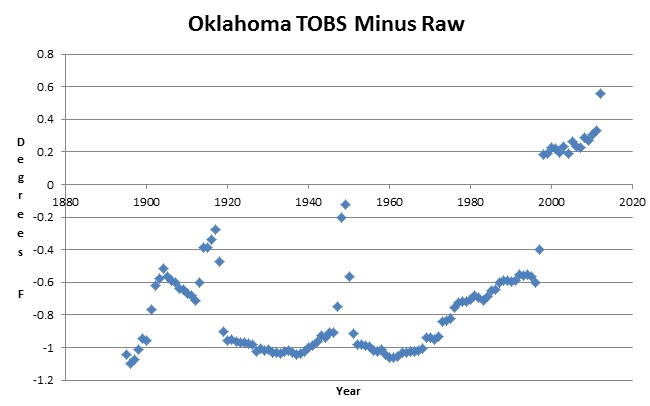
You can’t make this stuff up. USHCN2 bumped all Oklahoma temperatures up by 0.8 degrees between 1996 and 1998 due to claimed TOBS adjustments.
This is incredible to begin with, but particularly spectacular because they report no changes in temperature observation time, for any station in Oklahoma after 1994. The adjustment appears to be 100% fraudulent.


Hammer them Steven , thanks largely to you there are now many people becoming aware of the depths these guys will stoop to in order to fool people.
SMOKING GUN is right. There is no such thing as US Climate science anymore. It is apparently all voodoo now.
Reblogged this on Climate Ponderings.
So, Stephen, ever corrected the “CO2 falls as snow in Antarctica” meme, yet?..;)
Post the link where I said that.
Sure thing, bud!
http://wattsupwiththat.com/2009/06/09/co2-condensation-in-antarctica-at-113f/
What I said was
That is four degrees below the freezing point of CO2 and would cause dry (CO2) ice to freeze directly out of the air.
That statement is 100% correct.
At that partial pressure, the rate of sublimation is as great as the rate of rate of freezing, so there is no net accumulation. I never said anything about snowflakes.
Small correction: I said ‘snow,’ not snowflake.’ Secondly, your assertiont hat it “sublimate” is dead wrong, too, and this poster said it best [asterisks mine]. Point: You didn’t start the topic off this way:
“At that partial pressure, the rate of sublimation is as great as the rate of rate of freezing, so there is no net accumulation.” Need I remind you of how you did, and were proven incorrect?
“Unfortunately Steven Goddard got this one completely wrong.
CO2 phase diagram is very simple in comparison to water phase diagram. Please study it. It is all in that diagram.
The sublimation temperature of CO2 at 1 ATM is -78.5 C. At this temperature the vapor pressure of the solid CO2 is equal to the total pressure of the gas phase in contact with it which is 1 ATM. If the atmosphere would have 100% CO2, the pressure is 1 ATM and the temperature is -78.5 C, then an equal amount of CO2 is deposited as solid as is sublimed as gas. Things would be in balance. Again, if the atmosphere is 100% CO2, pressure is 1 ATM and the temperature is below -78.5 C, then more CO2 is deposited than sublimed (you are on the left side of the “solid-gas” line in the solid area in the phase diagram).
When the pressure of CO2 goes down, the sublimation temperature goes down too. That is the “solid-gas” line in the phase diagram. *With 385 ppm CO2 concentration the partial pressure of CO2 is so low that the temperatures needed for deposition to take place do not naturally occur on this planet.*
There is no freezing of CO2 below 5.11 ATM because liquid CO2 does not exist below 5.11 ATM. CO2 triple point is -56.6 C and 5.11 ATM. Below 5.11 ATM only solid and gas phases exist.”
Chemistry doesn’t lie, and rational scientists don’t forget those statements made by others with a political ax to grind. My point? Your words have often shown you to be,a t best, disingenuous, as regards your opposition to AGW work. If I’m also correct, this bit led to Watts banning you from his site….is that true?
Stop being an idiot and pretending that you know what you are talking about. Individual molecules know nothing about the partial pressure. That is strictly a statistical measure.
Below the freezing point, there are always going to be molecules switching from gas to solid. When the partial pressure is below the vapor pressure there is no net accumulation. When the partial pressure becomes equal to the vapor pressure, then ice starts to accumulate.
Your argument is identical to arguing that the freezing point of water depends on the humidity
Since you, like most of your ilk, cannot discuss w/o resorting to ad homs, I’m done. My point stands, and as a scientist, I think I DO know what I’m talking about. Enjoy the ban.
You started out the conversation with a sarcastic lie, and then you run away. Nice