http://math.nyu.edu/~gladish/teaching/eao/water-phase-diagram.jpg
A phase diagram represents a perfect equilibrium closed system. Such a system can not actually exist, because there are no impermeable materials and no perfect insulators.
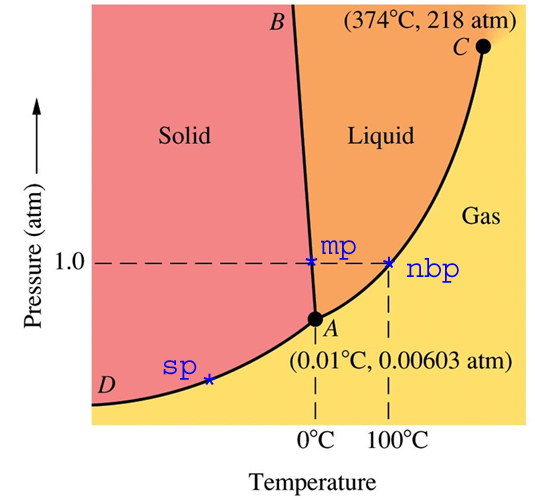
The phase diagram above represents an impermeable container (with zero thermal conductivity) containing pure water, filled to the brim and sealed.
For now, let us consider the case of one atmosphere pressure. At 1ºC, the container would contain only liquid water, no solid and no gas. At minus 1ºC, the container would contain only solid, no liquid and no gas. At 0C, (mp on the diagram) there would be a mixture of solid and liquid, but no gas.
Now, let’s slide to the right along the dashed line towards nbp. We get up to 90ºC (194ºF) and still no water vapour. There is no water vapour until we get to the boiling point at 100ºC.
Does this make sense? We all know that lots of steam comes off our pot of water before it boils. That is how a humidifier works. Does this mean that the phase diagram is wrong? Does it mean that our eyes deceive us?
No, it means neither. The phase diagram represents a closed system at equilibrium – which does not represent the real universe. It requires skill and experience to know how to use a phase diagram correctly.
Now, back in the real world. Imagine a field full of wet snow, complete radiative darkness, 100% humidity at 0ºC. The snow would theoretically remain on the ground forever, in equilibrium with the liquid and gas phases present. If the temperature went up to 1ºC, all of the snow would eventually melt and there would be only two phases – liquid and vapour. If the temperature instead dropped to minus 1ºC, all of the water would freeze, leaving only solid and vapour.
We can learn a lot from phase diagrams, if we understand how to use them – and what their limitations are in the real world.
———————————————————————————————–
When writing about the Arctic, I am talking about the real world of melting, freezing, sublimating and condensing water. I am not talking about pure academic theory, because nothing in the physical world works that way.


Hi Steve,
I like the example of ice cubes left in the freezer for an extended time. Even if the freezer never exceeds 0 C, eventually the cubes will sublimate out of the tray because of the finite vapor pressure. Thus, at 0 C vapor DOES exist in equilibrium above the water/ice.
People familiar with chemical engineering thermodynamics or physical chemistry know you’re correct about this, so your target audience is less informed. More basic examples might be appreciated by them. However, I don’t think that you trying to prove that you’re right will have any effect at all…someone else will need to show it.
-Scott
I’m sorry I missed out on the fray at WUWT, I would have support you on most points.
Your initial reference to the triple point wasn’t useful, as were some of your followup comments.
Of course water, ice, and vapor can coexist in an iso-thermal system around 0°, though phase diagrams don’t take into account the presence of other chemicals, e.g. salt, O2, and N2.