Some readers insist on denying the obvious fact that Venus temperatures are due to high atmospheric pressure. The latest argument being that the lapse rate is dependent on GHG levels. This is trivial to demonstrate as incorrect.
The first problem with that argument is that the lapse rate on Earth is very similar to Venus, despite very different atmospheres.
The second problem is that on Earth, water vapor is the most important GHG – yet the dry adiabatic lapse rate is independent of humidity (for unsaturated air.)
For an adiabatic process, first law of thermodynamics can be written as
ncvdT ? VdT / ? = 0
For adiabatic process:PdV = ? VdP / ?
Also since : ? = V / n and : ? = cp / cv we can show that:cpdT ? ?dP = 0
where cp is the specific heat at constant pressure and ? is the specific volume.Assuming an atmosphere in hydrostatic equilibrium: [ 8 ]
dP = ? ?gdz
where g is the standard gravity and ? is the density. Combining these two equations to eliminate the pressure, one arrives at the result for the DALR, [ 9 ]
In other words, the lapse rate is fixed on Earth – whether greenhouse gas levels are high or low.

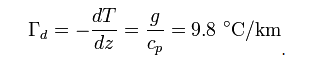

It is odd that some would deny science (lapse rate) to cling to an unproven theory.
Yes the differential form of the equation is as you state here: http://stevengoddard.files.wordpress.com/2011/04/screenhunter_15-apr-18-07-09.gif
Some elementary calculus gives:
T=T0-9.8z
T0 is the surface temperature and T is the temperature at height z, T0 is not determined by the lapse rate it is determined by the energy balance at the surface!
So you agree that lapse rate is independent of GHG concentration.
No it is not independent of the GHG concentration, the difference between the Earth and Venusian values is due to the different concentration of GHGs, 9.8 vs 10.5. That of course assumes that the Venusian atmosphere is an ideal gas which it isn’t, which makes a larger difference.
http://journals.ametsoc.org/doi/pdf/10.1175/1520-0469(1970)027%3C0219%3ATALRIT%3E2.0.CO%3B2
Did the content of this post completely fly over your head? I showed very clearly that lapse rate is independent of GHG concentration, but you fail to grasp the simple concept I presented.
The issue isn’t whether a lapse rate depends upon greenhouse gas concentrations (at least it not, from my corner), it’s about what causes the temperatures observed at the surface of the planets, as Phil indicates.
As I’ve asked twice now, and following on from your “experiments” on the WUWT thread:
…how would the temperature on Venus change?
The question doesn’t require a denial of the existence of lapse rates, or of the manner in which is is or is not altered by GHG composition. That gambit is merely a red herring.
Given that lapse rate is independent of GHG concentration, it is quite clear that lapse rate is independent of GHG concentration.
What part of that do you have difficulty understanding? It is fascinating getting a peek inside the bizarro minds of religious people.
Given that lapse rate doesn’t heat the system (it’s adiabatic, after all), what energetic inputs into a system, and what barriers to energetic output from a system, do cumulatively heat the system?
What part of that do you have difficulty understanding?
Are you completely daft?
Humour me, assume that I am completely daft if that spins your pinwheel, but please explain what energetic inputs into a system, and what barriers to energetic output from a system, do cumulatively heat the system?
Specifically, the Venusian platenary/atmospheric system; and if possible, with the alternative scenarios of the real, and the hypothetical 100% nitrogen, atmospheres.
I’m puzzled as to why you never answer these basic questions, even if you think that they’re daft or irrelevant. Does that mean that you don’t know the answer, or that you just aren’t saying?
The Sun heats the system, obviously. Are you completely daft?
stevengoddard says:
April 18, 2011 at 2:31 pm
Did the content of this post completely fly over your head? I showed very clearly that lapse rate is independent of GHG concentration, but you fail to grasp the simple concept I presented.
Actually you didn’t, Cp is dependent on gas composition, you chose to treat it as a constant however it isn’t.
You are throwing in very small effects as an argument against the order of magnitude failure of Sagan’s claim. It is funny watching alarmists scramble for crumbs.
You made a factually incorrect statement, I rebutted it. Given your reference to Venus it isn’t a ‘very small effect’.
Newton’s Laws are also factually incorrect, but we use them all the time because they are good enough for the real world.
Your attempts to obfuscate belie your lack of interest in the truth.
So, Steven Goddard, if Venus’ atmosphere was 100% nitrogen, but with otherwise the same essential mass/thickness, what would the surface temperature of the planet be?
So Bernard J, you are raising a straw man argument. There is enough GHG in both planet’s atmospheres to absorb essentially all of the LW radiation, and your question is irrelevant to this discussion.
We are discussing the real planets of Venus and Earth. Is it possible for you to stay on topic? Again,.your attempts to obfuscate belie a lack of interest in the truth.
Eh, I’m raising the strawmen?
You are on record as saying:
You quote Wikipedia, which said:
and to which you replied:
and also said:
So I ask yet again – if Venus’ atmosphere was 100% nitrogen, but with otherwise the same essential mass/thickness, what would the surface temperature of the planet be?
Complete crap strawman argument.
Why?
And does that mean that you don’t know the answer, or you just aren’t saying?
And for the record:
The nature of the temperatures on the “real planets of Venus and Earth” were apparently on-topically contemplated in your thread at Watts’ using four ‘experiments’ of exactly the same hypothetical nature as I proposed in my experimental question that you find so irrelevant.
In my own humble opinion the answer to my question is very much on topic.
Au contraire. It’s exactly my interest in the truth of what warms Venus that motives me to pursue this questioning.
If the atmospheric pressure at some point on the surface earth was the same as Venus, it would be extremely hot there. Anyone can see that, except for congenitally blinded climate alarmists.
Go camping in Death Valley in July, then try the same experiment on the top of Mt Everest. Report back what you find out.
Is this really such a difficult question to answer?
Let me put it this way. If the Earth’s atmosphere was the same composition as Venus’ atmosphere, but with the current earth atmospheric mass, and if I wore suitable breathing apparatus, would my camping experiences in Death Valley and on top of Everest be the same, temperature-wise, as they would be under current circumstances?
If I teleported to Venus and tried the same experiment there, on Maxwell Montes and on it’s lowest plain, and under the two atmospheric composition scenarios, how would the camping experiences differ again?
I see that question was too tough for you.
Try camping at the top of the Grand Canyon and at the bottom and report back to us. You probably won’t need any breathing apparatus and won’t need to raise yet another mindless strawman argument.
Steven Goddard.
I’ve spent many years of fieldwork climbing from valley bottoms to mountain ridges and back again, day and night, week after week, winter and summer. I am well aware of the effect of a lapse rate.
Perhaps we are arguing at cross-purposes here. I am not disputing the existence of lapse rates or of convection driven by the sun – to which, I acknowledge, you explicitly refer. I was hoping to get you to explicitly state too, that lapse rate determines the gradient of the distribution of heat through an atmosphere, rather than being tied (via atmospheric pressure) to being the source of heat through an atmosphere – the latter concept being one that many of your unquestioning readers have taken away from your commentaries, and which I think needs to be explicitly confronted.
My point is that if the entirety of gases in an atmosphere were transparent to infrared radiation then absorption of said radiation, and thus warming (and thusly re-emission to space), would only occur at the surface of the planet, and the atmospheric heating that leads to convection would diminish to the point where the lapse rate is not what it currently is.
Now I’m not an atmospheric physicist, but somewhere in the recesses of my memory I recall the term ‘isothermal atmosphere’ – an hypothetical idea, certainly, but one that serves to highlight the fact that atmospheric compositions vary in a manner that surely surely results in a variety of lapse rates, amongst other thermodynamic sequelæ such as ‘greenhouse’ activity.
I’m also not disputing that Earth and Venus have similar lapse rates. Nor am I subscribing to the ‘runaway greenhouse’ notion (which, frankly, I consider to be a red herring). What I am disputing is that atmospheric pressure/lapse rates aren’t the only things that determine the distribution of heat in an atmosphere.
This is why I have repeatedly asked you to get to the nuts and bolts of temperature in an atmosphere that is entirely nitrogen, compared with the actual Venusian one. I really don’t care if Venus’ thick atmosphere means that it would be very hot even without carbon dioxide – I want to know if you accept the conventional physics that says that a high concentration of carbon dioxide in a massive atmosphere will retain more heat than one without said carbon dioxide. And if so, by how much.
And the reason that I want to know this is because of the implications for Earth’s climate should we double carbon dioxide compared with pre-industrial atmospheric concentrations. And most especially if we should double it again after that, because then we are heading into the concentrations that, in geological history, saw an ice-free planet, with acid, anoxic oceans, and with an ecology staggeringly different to the one in which humans evolved.
Is it possible to sensibly discuss these issues?
Bernard,
I appreciate your concern. However, this post simply showed the math for lapse rate, and GHG concentration does not affect it. It is also abundantly clear that if there were a place on earth at 5km below sea level, it would be extremely hot. I just don’t see that this is in any way controversial.
Though i am hardly qualified to make a learned comment on this topic, as a free flight pilot I have experienced high humidity days with no thermal activity and low humidity “blue thermal” days with thermals all the way to heaven. So what do green house gases have to do with laps rates?
Exactly. But you probably don’t smoke as much weed as Sagan did.
ahahahahahahahaha!!
rotfl
OK–how can one reconcile the assertion that the energy heating Earth and Venus comes from isolation (made elesewhere by SG) with the assertion that pressure completely determines temperature? (That is what is being asserted by this (mis)-use of the ideal gas law.)
Do tell.
Read the article and tell me what you disagree with and why. The endless attempts to raise strawman arguments show how desperate your side is.
I did read it.
Your use of the ideal gas law implies that atmospheric temperature are determined by pressure. You reinforce this viewpoint explicitly in several comments–for example, 4/18 at 5:13 PM, below:
“. . . it is the pressure of non-GHG gasses which keep Earth’s temperature up.”
This leaves no room for insolation to affect temperature, which is counterintuitive, to say the least. I didn’t raise any new issues here; I simply asked you to reconcile this idea about pressure with statements you have made elsewhere–for example, 4/18 at 4:13 PM, above:
“The Sun heats the system, obviously.”
Frankly, I don’t see how these statements can be reconciled, and therefore I don’t see you presenting a coherent picture of how atmospheres react to solar heating.
The Ideal gas law has four variables. You obviously need all four to balance the equation. If you don’t understand basic math, why post?
And which of the four variables can be used to handle solar radiation in an atmosphere?
If you can’t answer a simple question, why write a post?
The four variables of the equation are P,V,n and T. I’m not planning on writing a unified theory which ties everything in the universe together.
Oddly enough, I already knew what the four variables were.
I’m not asking for you to tie everything together; just the two things you’ve said affect the warming of planetary atmospheres: pressure, and solar radiation.
Come on–not even a little hint for us poor small minds who fail to grasp your thought?
I have quite clearly stated and Tamino quoted that essentially all of the heat in the system is due to the Sun. If you can’t grasp that simple concept then please stop wasting my time.
Well, I think that I do grasp that all the heat in the system comes from the sun–whether “system” refers to the solar system or a particular planetary atmosphere.
What I don’t grasp is the connection you assert with this heat and the lapse rate as your source derives it. You say that temperature depends on pressure, and use the ideal gas law to justify this. Yet there appears to be no obvious way that the ideal gas law allows us to calculate the effects of solar heating.
I even consulted:
http://abitabout.com/Adiabatic+lapse+rate
which you had kindly credited as the source of your derivation of the lapse rate.
I found that the derivation was specifically for the “dry adiabatic lapse rate,” about which the article said:
“The term ‘adiabatic’ means that no heat transfer occurs into or out of the parcel.”
So if the equations you used apply to situations where there is no heat transfer, and planetary atmospheres–as we all agree–do receive energy (heat) from the sun, then what are you saying? How does atmospheric pressure affect the incoming solar radiation, exactly, in order to create such an elegant correspondence as you describe?
P. S. I’m very sorry if you feel that I’m wasting your time. I had thought that you were taking the time and energy to run this blog in order to educate us all about climate science?
Sorry, we get a number of people here who’s sole purpose is to obfuscate and waste time, and it is difficult sometimes to differentiate.
Appreciated.
Candidly, you might call me an “alarmist” on AGW, since, I am indeed “alarmed” about it–the consequences for pretty much everything I hold dear would be very negative if the IPCC were correct. It would be a great relief if I could stop fussing over this issue.
However–and being candid once again–I’m not very reassured by the present post. As I posted to omnologos, below, the dry adiabatic lapse rate currently seems to me to have little relevance to the question of whether there is a greenhouse effect or not.
But I suppose that we’re well past the point of going round in circles about it.
Do you doubt that it is hot in Death Valley?
?
If the composition of Venus’ atmosphere of 96% CO2 causes the “excess heat” what then explains Mars at 95% CO2 with no excess heat? I’d say pressure what say you.
Percentages being near equal don’t mean the actual compositions are near equal. In terms of absorption, consider Beer’s Law. The concentration term is not in percentage, but in a unit reflective of molecules/volume.
-Scott
moles and volume
Mkelly.
You, as so many others have, are confusing correlation with causation.
You are confusing the cloud in your head with reality.
Bernard, that’s beautiful. Kudos to you! Alarmists, don’t often recognize that fallacy trap.
The concentration of CO2 in the Martian atmosphere is about 10,000x lower than Venus, that coupled with very low line broadening greatly lowers the GH effect on Mars.
The density of CO2 in Mars atmosphere is about 10X higher than on Earth, yet Mars is very cold. This is due to the low atmospheric pressure.
Of course, dQ=0 for radiative (non-adiabatic) processes too 😉
Ask a diesel engineer what temperature he would expect with a 90x compression ratio. It will be very close to the surface temp of Venus.
Ask a diesel engineer what temperature he would expect with a 90x compression ratio, if the pistol froze in place at compression and no gases were exhausted from the cylinder, and the whole was left in exactly that state left for an hour.
Your example only works for circumstances described by the Kelvin–Helmholtz mechanism, such as the ongoing gravitational compression of the Jovian atmosphere. Venus is not experiencing a Kelvin–Helmholtz compression, so the comparison with a piston is spurious, as I tried to indicate on an earlier thread.
Yes it is. Convection is constantly compressing and heating downward moving gas, and cooling upwards moving gas.
Your failure to understand the most basic concepts of the atmosphere is why you are unable to follow the discussion.
And the actions of convection that you describe are adiabatic, and hence to not account for the total thermal energy in the system.
So, trying to elicit an answer to the ‘Experiment 5’ question yet again, will “[c]onvection… constantly compress… and heat… downward moving gas, and cool… upwards moving gas”, to give an equilibrium planetary surface temperature range that is the same, whether the gas in the atmosphere is 100% nitrogen or a contemproary Venusian mix?
I am not going to waste time on your strawman questions. Why are you incapable of focusing on the real universe and the topic at hand?
stevengoddard says:
April 18, 2011 at 2:57 pm
Newton’s Laws are also factually incorrect, but we use them all the time because they are good enough for the real world.
Your attempts to obfuscate belie your lack of interest in the truth.
It’s exactly my interest in the truth that causes me to rebut your incorrect statements.
No one would use the Earth’s lapse rate for the Venusian atmosphere, not only is there an approximately 10% error even in the ideal gas one, but if the real gas lapse rate is used then the error is larger.
Whether or not your statements are correct, they in no way change the fact that Sagan’s claims were incorrect,
Whether Sagan’s claims were correct or not has nothing to do with this thread. My claims are correct, yours are not, now that is relevant.
Phil, this may come as a shock to you, but nothing in the real world is exact. That is why we have engineering tolerances. You can nickel and dime yourself into complete stupidity if you try hard enough.
Of course, Mercury has virtually no atmosphere, which means its temperature must be absolute zero 🙁 Since sunlight obviously doesn’t matter
Thanks for proving my point. The density of CO2 is 10X higher on Mars than on Earth, yet the temperature on Mars is much lower.
As you so eloquently stated, it is the pressure of non-GHG gasses which keep Earth’s temperature up.
Yes, and birds fly because they aren’t subject to gravity.
Steven Goddard.
As you seem reluctant to answer my question about a 100% nitrogen atmosphere on Venus, perhaps you will indulge me in an alternative question.
Using the non-reflected total solar irradiance per square metre on Venus, and its atmospheric pressure/thickness and lapse rate, show the worked calculation that demonstrates that this is all you need to determine the surface temperature, as you have been claiming for so long.
By doing so you should be able once and for all to prove that carbon dioxide is not responsible for the high temperature of Venus.
Bonus points for proving that replacing carbon dioxide with nitrogen doesn’t change the result…
I think I have shown already that if you have lapse rate and tropospheric thickness, all you need to calculate the surface temperature is the tropopause temperature, with a lower limit easily established by setting the tropopause at 0K.
Next!
It is very easy to understand.
The temperature of a gas is equal to the average kinetic energy of each molecule multiplied by the number of molecules. Now, it is physically impossible for gas molecules to consistently maintain a higher average speed than the surrounding molecules. Yes, I know, warm gases go up. But their molecules can’t consistently maintain a higher speed.
If molecules at a high altitude had a consistently higher kinetic energy, what do you think would happen when they hit lower altitude molecules? The lower altitude molecule would be bumped harder and the center of mass of both molecules would move toward the ground. So the lower altitude would be compressed. So we conclude this:
If the lower pressure of a planet is stable, than the kinetic energy per molecule of gas is independent of altitude.
Now if you accept all planets have a pressure inversely proportional to altitude, you have to conclude that temperature is inversely proportional to altitude.
Marc,
One wonders why pressure drops exponentially with altitude, while temperature drops less rapidly? For that matter, why is the temperature about 90x at the surface of Venus (compared to Earth), while the temperature is only a factor of 2-3 hotter…shouldn’t it be 90 times hotter?
I did mean pressure is 90x higher *
I would say that Venus atmosphere filters a lot more sun energy than the Earth. That would explain why the kinetic energy of low altitude molecules can be lower.
Venus has a very high albedo due to the thick cloud cover. The amount of sunshine getting through the cloud cover is about the same as earth. That is why Venus is so bright.
I have made a small error in my reasoning, since gravity adds kinetic energy to molecules traveling down, the kinetic energy of low altitude molecule has to be slightly higher than proportionally to the variation of potential gravitational energy.
So the temperature is still inversely proportional to altitude but the variation is greater than what I have shown before.
Meteorologists have known this for quite some time. I wonder why they never shared their insights?
http://www.engineeringtoolbox.com/air-altitude-temperature-d_461.html
Because everyone already knows about the lapse rate. It’s not new stuff. Come to our Atmos. Science program in Wisconsin, and you’ll go through a very detailed treatment of it in your junior year in atmospheric thermodynamic.
Hey, how are those non-warming winters coming in Wisconsin?
Atmospheric composition and absolute temperature does have an effect on Cp, and therefore the dry adiabatic lapse rate. So does the gravitational constant g. Its interesting to look at planets other than Venus and Earth.
Venus g= 8.9 m/s^2
Earth g= 9.8 m/s^2
Jupiter g= 24.3 m/s^2
Cp (Earth, lots of N2) = 1.0 J/g K
Cp (Venus, lots of CO2) = 0.8 – 1.2 J/g K, depending on temperature.
Cp (Jupiter atmosphere, lots of H2) = 12.4 J/g K
Earth DALR = 9.8 C/km
Venus DALR = 7.4 – 11 C/km ( depending on temperature)
Jupiter DALR = 2.0 C/km
Jupiter has the hottest ‘surface’ temperatures, about 10,000 K, of any planet in our solar system (where the Jovian ‘surface’ is defined at the transition to metallic hydrogen).
I still expect to soon read the CACA rants about the high methane concentrations (3000 ppm!!!!!) in Jupiter’s atmosphere that have caused runaway Jovian GHG surface warming…
I am glad the lapse rate is independent as I would hate to have another revolution on our hands.
I am surprised that CO2 has not filed a defamation suit against the Climatologists and the Chicken Little Brigade that that repeats their fantasies! It is as if these folks are attempting to destroy the credibility of one of nature’s hero gasses that are necessary for life to exist on this planet! This is worse than claiming the Easter Bunny does not lay pretty colored eggs!
Steven:
For the quality of responses you are getting on this thread you could just as well be discussing the fertility festival this week end!
stevengoddard says:
April 18, 2011 at 6:23 pm
Phil, this may come as a shock to you, but nothing in the real world is exact. That is why we have engineering tolerances. You can nickel and dime yourself into complete stupidity if you try hard enough.
The 10% that you continually dismiss is not an ‘engineering tolerance’.
The runaway greenhouse effect which Sagan talks about is not a 10% effect. That is the whole point of this conservation.
No the whole point of this thread is your assertion that “The Lapse Rate Is Independent Of Greenhouse Gas Content”, which is wrong. Sagan doesn’t come in to it!
But Phil, Sagan is the inspiration for decades of runaway greenhouse bullshit.
Well he appears to be responsible for some from you here! I know you desperately want to change the subject from the lapse rate because you’re taking a beating on the science but Sagan has nothing to do with this thread.
You are cracking me up Phil
BTW – Mars is only 1.5 AU from the Sun (not 2 as you claimed) and it doesn’t have any clouds, so the radiation reaching the surface isn’t tremendously different from Earth. Not close to the 4X you claimed.
The Bond albedo of Earth is 0.29 vs 0.25 for Mars, try again
Here, this might help.
Earth 0.9833 – 1.017 —- 1,413 – 1,321
Mars 1.382 – 1.666——- 715 – 492
Distance is in AUs(the distance between earth and the sun.) and energy values are in w/m2 for the isolation.
Imars/Iearth= d2 earth/d2 mars = 1(sq)/1.524(sq) = 0.431
Before this gets out of hand, let me claim precedence on the Venus Question (first publication date: Aug 17, 2007), that will henceforth be known as solved by the Morabito-Goddard Answer.
See you all in Stockholm! (or Oslo, as these things happen)
lol, you may have to wait for a bit, but I’ll petition for you guys!!!!
So I guess my comment on your botched derivation was deleted…
Nothing has been deleted
Are they employing a new tactic?
This always ends up in convoluted exchanges when the basics are pretty simple.
Since by removing GHGs (H2O excluded) the adiabatic lapse rate doesn’t change, we can definitely assume that GHGs have little to do with the adiabatic lapse rate.
Since the only meaningful way to compute a planetary temperature when there is an atmosphere, is by starting at the tropopause and then going down to the surface using the adiabatic lapse rate (as going the other way around could lead to physical impossibilities such as negative temperatures in K), we can definitely assume that the only possible contribution of GHGs to a planet-with-atmosphere’s surface is in modifying the height of the tropopause.
Can we agree on both propositions? Please explain why not. And by the way the consequence of the above is that the one clear, smoking-gun proof of GH-led global warming/climate change would be found in a general change in the height of the tropopause. Does anybody know if that’s been found already?
“a planet-with-atmosphere’s surface” TEMPERATURE, of course…
Well, let’s try this one on for size. The “abitabout.com” article providing the lapse rate derivation Mr. Goddard used in the post says that, according to the International Civil Aviation Organization:
“From 11 km (36,090 ft or 6.8 mi) up to 20 km (65,620 ft or 12.4 mi) , the constant temperature is ?56.5 °C (?69.7 °F).”
So, 11 km x 9.8 degrees K/km gives a dT of 107.8 C. That sounds OK, until we run the numbers a bit more. The tropopause temp, -56.5 C, translates to 217.5 K. Add 107.8 to that, and we have a surface temperature of 325.3–that’s 52.3 C, or 126 F!
I think we can all agree that if that were the global mean temp, we would be in serious trouble!
You find that if you consult the article, though, that the actual measured atmospheric temperature gradient does not follow the dry adiabatic lapse rate. Rather, there is an entity called the “environmental lapse rate.” The ICAO uses a value of 6.49 K/km for the tropospheric environmental lapse rate.
All of that leads me to conclude that your second “proposition” is not demonstrated, since (from all considerations mentioned here so far, at least) we don’t know what determines the environmental lapse rate.
However, there is some evidence of a rising troposphere:
From 2003–
https://www.llnl.gov/str/March04/Santer.html
When you get to an altitude where the air is saturated with H2O, the lapse rate drops off to the moist rate which is lower.
Kevin – in the troposphere, the temperature profile generally follows the line defined by the adiabatic lapse rate. I do not think there is any discussion about this. And if anybody wants to show that the a.l.r. is affected by GHGs, I am sure they could easily devise an experiment to prove it.
As for the Santer link, it proves nothing about a “rising troposphere” (I think you mean, “tropopause”) and the (a) diagram in the last figure in that page pretty much disproves the whole point. BTW the claimed 200 meters in height increase translate into .2*9.8=1.96C or .2*6.49=1.3C of additional surface temp between 1979 and 1999. Observations do not agree, to say the least.
You asked about studies on this; I provided the link; you don’t accept it. Fine.
(And of course you are correct; I should have typed “tropopause” rather than “troposphere.”)
But my main point stands–I don’t agree with your second proposition because:
1) The dry adiabatic lapse rate doesn’t control the environmental lapse rate–“generally follows” doesn’t cut it.
2) “Back radiation,” far from being a modeled construct first, has a long history of actual measurement behind it–for example, Guy Callendar cited (IIRC) one study on this from 1918. Certainly, there have been many modern observational studies of this, including longitudinal ones demonstrating a change reflective of increased GHG concentrations.
Since radiation to the ground from the troposphere is, ipso facto, a GHG “contribution to the surface,” I think your proposition is empirically falsified.
omnologos,
Goddard’s derivation can’t say anything about how GHG’s may or may not affect the lapse rate because he turned off radiation in his derivation in the very first line (radiation is not an adiabatic process, so the RHS side is not zero). Think UV absorption by ozone setting the stratosphere inversion. He can’t prove something doesn’t have an impact if you assume it isn’t important to begin with.
In a no-GHG atmosphere the emission level IS the surface level, and no layer can rise above the effective temperature set by the absorbed incoming solar radiation.
Pages 299-300 of Raypierre’s book explain where and when radiative effects can be disregarded.
chriscolose, just to set the record straight, Mr. Goddard doesn’t claim the derivation–it is taken from the “abitaboutit.com” article on “lapse rate.” He gives the link for it in the post.
That is also the source I used for information about the environmental lapse rate, mentioned in my response to omnologos.
But I agree–you can’t successfully use an assumption to prove itself. And it appears to me–mathematically unsophisticated though I undoubtedly am–that that is what the present argument by Mr. Goddard tries to do.
That’s partly why I kept asking about how insolation could be handled by the ideal gas law. I’d have liked an answer, but never got one.
Sorry, “abitabout.com.”
There is no question that a Grand Canyon 50 km deep would be incredibly hot. Why do alarmists continue to avoid this central topic?
He also assumes that Cp is independent of the gas composition which it isn’t, if you write instead Cp(T, composition) then ?d is also a function of T and gas composition. Of course since Steve blindly copied the derivation he didn’t know that.
You are nitpicking about minor details while ignoring Sagan’s order of magnitude error.
stevengoddard says:
April 20, 2011 at 2:25 pm
There is no question that a Grand Canyon 50 km deep would be incredibly hot. Why do alarmists continue to avoid this central topic?
Because it’s an irrelevant distraction not a central topic, if the Earth’s radius was 50 km smaller it wouldn’t be ‘incredibly hot’!
It is exactly the point. The hot temperatures in Venus are primarily due to the thick atmosphere – not the CO2. Sagan was wrong. That is the only reason we are having this discussion.
No the hot temperatures on Venus are primarily due to the fact that the atmosphere is optically thick, which is due to the composition. Pressure broadening of the absorption lines is important but couldn’t occur if it weren’t for the CO2, it wouldn’t be so in a N2 atmosphere.
Your belief that Sagan was wrong is based on your lack of understanding of the science.
You will do anything to avoid admitting the obvious fact that Earth would be nearly as hot at the same pressure. Why the intellectual dishonesty?
stevengoddard says:
April 20, 2011 at 2:37 pm
You are nitpicking about minor details while ignoring Sagan’s order of magnitude error.
Which is?
stevengoddard says:
April 21, 2011 at 1:16 am
You will do anything to avoid admitting the obvious fact that Earth would be nearly as hot at the same pressure. Why the intellectual dishonesty?
There is none, it’s not a fact nor is it obvious.
This is very, very tricky math. 9.8C/km * 50km = 480C
Beyond the grasp of an Ivy Leaguer.
Such tricky math that you got it wrong!
9.8ºC/km * 50km = 490ºC
The relevance of it escapes me though, so the temperature at the bottom of a 50 km canyon would be ~500ºC.
This is amazing. Colose points to Raypierre’s book. I read the troposphere chapter of that book, and report here (but anybody can read it in Amazon) that Raypierre is adamant on not considering radiative effects in the troposphere (as first approximation). Then we’re back here talking about what? Radiative effects in the troposphere!!
This is amazing indeed.
lol, yes, the amazing circular discussion.
Steven Goddard.
I’m curious to know what your response is to the ongoing discussion of Venus’ atmosphere, of its pressure and temperature, and of what effect the composition of the component gases has. The discussion is typified here:
http://ourchangingclimate.wordpress.com/2011/06/07/venus-climate-co2-greenhouse-effect-density/
Of particular interest to me is the conclusion that people make about a Venusian atmosphere composed entirely of nitrogen, and how this compares to your own perception of the matter. Bart’s final paragraph might be a place at which to start…
It is ridiculous to discuss an atmosphere containing no CO2 or H2O. A more sensible boundary condition is an atmosphere with a small component of H2O and CO2. Even at -100 degrees, the amount of H2O in the atmosphere is substantial.
“It is ridiculous to discuss an atmosphere containing no CO2 or H2O.”
It’s not ridiculous if one it attemtping to attribute atmospheric heating to the ‘greenhouse’ effect, as opposed to, say, straight pressure ‘effects’ or to lapse rate.
Consider the very first sentence of your post at the top of this thread:
“Some readers insist on denying the obvious fact that Venus temperatures are due to high atmospheric pressure.”
Consider too Bart Verheggen’s comment:
“It seems that you [JeffID] agree that the high temperature on Venus is due primarily to a strong CO2 greenhouse effect (few hundred deg) and secondarily (?) to the high surface pressure (the ~80 deg number that was mentioned upthread).”
and that fact that JeffID did not dispute it.
So, getting back to the whole original question, how much of the thermal energy in Venus’ atmosphere really does ‘come from’ the pressure of the atmosphere itself, and how much is a result of the composition of the gases in the atmosphere?
If changing the composition from carbon dioxide (and water, and other GHG) to nitrogen results in a drop in atmospheric temperature of several hundred degrees celcius, when pressure remains the same, then is not the fundamental premise that pressure is the ’cause’ of the heat flawed?
In order to produce adiabatic heating, there has to be a mechanism to produce convection in the atmosphere. Heating of the lower layers of the atmosphere is essential for convection to occur.
And as you noted on 18 April 2011 at 4:13 pm, “[t]he Sun heats the system, obviously.”
Whether the Venusian atmosphere is 96% CO2 or 100% nitrogen equivalent, the pressure (by definition of the equivalence) is the same. And yet, according to many, the temperatures of the two atmospheres would be different. The incoming radiation flux would be the same, so if those people are correct, is it not the composition of the gases that is the primary determinant of the difference in the two temperatures?
To revisit the first sentence of the thread again:
“Some readers insist on denying the obvious fact that Venus temperatures are due to high atmospheric pressure.”
All I am trying to acertain is to what extent atmopheric pressure (as opposed to gas composition) is responsible, according to your model, for the temperature of Venus’ atmosphere.
The lapse rate is very similar on Venus and Earth. That should answer your question.
A credible answer to my question is whether Bart Verheggen is correct in his comment:
“Quick rundown on Venus’ climate:
Venus is closer to the sun than the Earth, but its higher reflectivity more than compensates for that. Without a greenhouse effect Venus would actually be colder than the Earth would be without a greenhouse effect. In reality Venus is about 500 degrees warmer than this so called black body temperature (the greenhouse effect on the Earth is about 33 degrees). This is primarily due to the inception of infrared radiation by its thick atmosphere of almost pure CO2. The high density also helps, but is of secondary importance.”
No, his statement is ridiculous. If you replaced Venus atmosphere with one of similar composition to Earth, it would be similar temperature as it is now. We know that because the lapse rate is very similar.
I have been racking my brain for years now, trying to understand what is missing in the minds of GHG believers, that prevents them from comprehending the interdependence of pressure and temperature in the physical behavior of gasses; so that I might explain it in a way they might understand. All of my attempts have failed, although at one point on Tamino’s site, Kevin McKinney seemed to show some sign of comprehension. But, that was quickly overtaken by Timothy Chase’s obfuscations, and accusation that I am “merely playing stupid”.
The simplest explanation I have seen is that the energy in a gas is the sum of its kinetic energy, potential energy, and internal energy. The kinetic energy is akin to the velocity of the molecules, the potential energy to the height of the gas, and the internal energy to the spin. Temperature reflects the rate of molecular collisions.
The adiabatic lapse rate dependency on pressure is not some new hypothesis proposed by Steven Goddard, it is the long established conclusion when first law of thermodynamics is applied to gas held about a sphere by gravity. It certainly predates the Sagan-Hansen GHG hypothesis as the explanation for Venusian surface temperature.
There have been several lines of argument showing how the GHG hypothesis fails to explain surface temperatures of Venus or Mars. But, they are ignored or get countered by unquantifiable line broadening hypotheses. or similar.
I am not playing. I don’t like to think anyone is stupid. At times my dog even appears smart. But I really have to wonder why so many go to such great lengths to ignore the physics of gas behavior.
I think this topic needs more consideration. For instance this paper by Verkley and Gerkema is relevant:
“”A column of dry air in hydrostatic equilibrium ….. bounded by two ?xed values of the pressure, and the question is asked, what vertical temperature pro?le maximizes the total entropy of the column? Using an elementary variational calculation, it is shown how the result depends on what is kept ?xed in the maximization process. If one assumes that there is no net heat exchange between the column and its surroundings—implying that the vertical integral of the absolute temperature remains constant—an ISOTHERMAL pro?le is obtained in accordance with classical thermodynamics and the kinetic theory of gases”
http://www.nioz.nl/public/fys/staff/theo_gerkema/jas04.pdf
If that would not be the case, it would be possible to build a machine that makes mechanical energy from a single source of heat. This goes against the 2nd law of thermodynamics.
The natural intuition that the temperature must be warmer at the bottom, because molecules gain energy, when falling, is wrong because it’s exactly compensated by another phenomenon: Those molecules that have little energy cannot go up as well as those with more energy.
This is not to deny that the sudden imposition of a gravitational field on a column of gas would
indeed set up a temperature gradient. It would – but it wouldn’t persist. It would
quickly be homogenized and the column would become isothermal.
So if a column of gas behaves this way would a GH free atmosphere behave the same way too? I think it might. Without the driving force of IR radiation from the upper reaches of the atmosphere there would be very little net heat flux in our column of air. There would be no re-radiation of course and very little convection. A GH free atmosphere would be very different from our present one and approximately much more closely to an isothermal state.
Convection is the only thing that matters in the troposphere …oh, nevermind that
Convection is important, sure. Convection currents carry heat to an altitude of several kilometres and from there some of that heat is radiated out into space which causes the air at altitude to cool.
But if there were no GH gases in the atmosphere the heat would just stay there. It would have nowhere else to go. Therefore it would be a lot warmer.
So the lapse rate isn’t independent of greenhouse gas content, after all!
Can we progress this by distinguishing between the environmental lapse rate and the adiabatic lapse rate ?
Clearly the latter is pressure dependent but the former is not since the surface temperature and the slope of the temperature decrease from surface to tropopause can vary for many reasons locally and regionally and possibly globally if the oceans vary their rate of energy release or if global cloudiness changes occur.
So how about the environmental lapse rate being affected by GHGs but by the time one gets up to the tropopause the effects of evaporation, condensation and rainfall have smoothed out the environmental lapse rate to match the adiabatic lapse rate.
So one could find GHGs giving a steeper slope from the surface to tropopause but the normal adiabatic lapse rate upward from there.
Whoops, I forgot that the stratosphere warms with height so scrub the last sentence.
Anytime I read a thread on this topic it seems there are people who get hung up, conceptually, long before they get to the math. The hang up seems to go like this, “Pressure increases can’t cause temperature increases.” When I think about the lapse rate I think of it differently because I agree that increasing pressure doesn’t ‘create’ higher temperature in anything more than a transitory manner. Sure there may be some effects from diurnal pressure changes but overall they can’t justify the observed gradients on their own, can they?
I like to think of it more like this…. Higher pressures ‘support’ higher temperatures.
If you isolate a column of air extending from the surface upwards it will have a pressure lapse rate totally dependent on mass. If you further assume that the column isn’t exchanging energy horizontally then it is solely the energy content of each, say, meter of gas that is holding up all the gas above it. From these assumptions isn’t it reasonable to assume that there is an energy lapse rate in the column that is dependent on mass? It’s also true that there is a density lapse rate dependent on mass of the column.
Now each meter in the column will always (in practical terms or at least as an initial assumption) have the same differential pressure measured on its ends regardless of its temperature. If you heat the column at the bottom, the gas in that first meter will get more energetic. It will want to increase its differential pressure but it can’t because the column will simply expand upwards. So what happens? Well its density goes down. Each meter has a constant differential pressure, a constant volume and a variable number of molecules. So this energy is transferred all the way up the column. Each one meter chunk has to expel some molecules (upward, they’re going to seek lower pressure) as they get more energetic. Each meter gets less energy than the one below it. Each meter gets lower density.
If that column had an ideal gas in it with absolutely no radiative absorbtion qualities you have little one meter chunks of constant pressure, constant volume, and a molecular lapse rate. Yielding a temperature lapse rate totally dependent on the mass of the column, no?
Changing the column to nitrogen would be a pretty close approximation to ideal and nitrogen has very low radiative absorbtion. You would still get this lapse rate as long as you keep the heat on. You do have to keep the heat on because this descriptive process is NOT in equilibrium. There’s heat being applied at the bottom all the time. Take the heat away and each meter will eventually acquire enough molecules at appropriate energy levels to level out its temps.
In fact I would conjecture that a column in equilibrium would in fact be isothermal (no temperature lapse rate) and that is a large part of why these threads go haywire. The fact that it is not in equilibrium (heat at the bottom) is what causes the mass dependent temperature lapse rate.
Why is it hot in the centre of planets?
“Some readers insist on denying the obvious fact that Venus temperatures are due to high atmospheric pressure.”
The insistence is because they’re right. The adiabatic lapse doesn’t raise any temperatures higher than they would be otherwise. All it does is creates a thermal gradient starting at the S-B surface equilibrium temperature and becoming progressively cooler with increasing altitude. altitude. Energy remains equally distributed, on average, among all molecules. Kinetic energy is replaced by an equal amount of gravitational potential energy as altitude increases.
Venus’ surface is hotter than S-B equilibrium temperature because it’s atmosphere is so dense it alters the thermal gradient between the planet’s molten core and the surface. Internal heat from planetary formation and radioactive decay is on the order of 100mW/m2 on the earth and Venus. If you can insulate sufficiently 100 milliwatts over long spans of time will build up to a very high equilibrium temperature. On the earth when this internal heat reaches the surface through the insulating layer of crustal rock it quickly dissipates because there’s only wonderfully convective ocean water and/or thin transparent atmosphere blocking its escape to space. On Venus there is no water only an extremely dense atmosphere, a surface that receives no light from the sun, and with little or no convection below the cloud layer which blots out the sun.
Blah blah blah blah
Dis it occur to anyone that the formation of Venusian clouds from SO3 and water vapor has anything to do with the heat near the surface?
Any idea what the heat of that reaction is?
Would Dale Springer or anybody else please compute what the Earth’s surface temperature would be if there’d be a tremendous outgassing increasing the atmospheric mass by 90 times, and leaving the composition unvaried?
(hint: it would be MUCH MUCH MUCH more that it is now)
“If you can insulate sufficiently 100 milliwatts over long spans of time will build up to a very high equilibrium temperature.”
Amazing! With this discovery we will have no need to heat our ovens and homes? All we need is to insulate and reflect the heat back in and the temperature will keep on climbing!
Anyway, only 170W/m^2 hits the surface of Venus and you cannot use your theory to explain why the poles (high pressure zones) are hot so this bloggers facts stand on solid ground.
Stephen Wilde says:
September 28, 2011 at 8:58 pm
“Can we progress this by distinguishing between the environmental lapse rate and the adiabatic lapse rate ? Clearly the latter is pressure dependent.”
The lapse rate in a non-convective atmosphere is established by air mass, gravitational constant, and heat capacity of the gas mixture. Saying it’s established by pressure alone seems to be overly lacking in critical detail.
What needs to be made clear is that pressure does not establish temperature in this ideal atmosphere. Pressure and temperature are disconnected by the ideal gas law in this case. When the column is warmed its pressure does not increase but rather its volume expands. There are two general applications of the ideal gas law. One where volume is constant (a closed rigid wall vessel) and one where pressure is constant (an open vessel). An atmosphere is the second type – it’s open at the top.
Therefore, because increasing temperature does not increase pressure in this case, increasing pressure does not increase temperature in this case. It also simply increases the volume. Equilibrium temperature is not effected by pressure.
Time to revive this thread with this, which is bit on the long side but simple:
No gaseous atmosphere can ever cause the surface temperature beneath it to fall below the S-B temperature.
The reason being that energy transmission through a gas by way of conduction and convection is always slower than direct radiation.
For a gas to prevent the S-B temperature from being reached it would have to cause energy to flow through faster than radiation which is impossible.
What then of albedo ?
It turns out that albedo only affects the distance that the surface temperature can rise above that predicted by the S-B equation.
The surface temperature enhancement will therefore be higher for a transparent atmosphere than for an opaque atmosphere.
The reason being that more conduction (to the gases from the surface) can occur if a greater proportion of the incoming energy reaches the surface.
The more reflective is the atmosphere the less radiation will reach the surface, the less conduction will occur and the smaller the surface temperature enhancement will be.
For a fully opaque atmosphere there would be nothing reaching the surface, no conduction from surface to atmosphere and no thermal enhancement.
Radiative theory proposes that the convection resulting from conduction has a net cooling effect but I have shown in my earlier post why there is no net surface cooling from adiabatic circulation.
The reason is that all energy uplifted adiabatically comes back down adiabatically.
It therefore follows that adiabatic convection has no effect on the amount of surface thermal enhancement.
Convection (in so far as it is adiabatic) neither warms nor cools the surface
The sole determinant as to how high the surface enhancement can rise for a given mass of atmosphere is albedo and the enhancement is highest at full transparency.
Radiative theory proposes that greater atmospheric opacity has a cooling effect due to reflective capability and so it does, but only down from the higher surface enhancement that could have been achieved with full transparency.
The fact that the surface enhancement is greater if the atmosphere is transparent ‘proves’ the dominance of mass as the cause of that surface enhancement.
Only if the maximum amount of conduction can occur from surface to atmospheric mass can the maximum thermal enhancement at the surface be achieved and the maximum amount of conduction occurs with full transparency.
It follows that with maximum transparency and maximum surface thermal enhancement there will also be maximum convection.
As I said many times previously, you have to have maximum convection in a transparent, non radiative atmosphere in order to get energy back to the ground fast enough for radiation to space to match incoming radiation.
Introducing radiative capability reduces transparency, reduces the surface thermal enhancement by reducing total conduction from surface to atmosphere and does not require such a vigorous convective circulation to achieve radiative balance with space.
The reality is that transparent non radiative atmospheres warm surfaces more than opaque atmospheres and radiative, gases by introducing opacity, cool surfaces below that which would have been achieved in their absence.
Both of those propositions are the opposite of radiative theory.
It also follows that the greater is the atmospheric mass the more conduction there will be and the higher the surface enhancement will rise at a given level of atmospheric transparency.
The surface temperature is a result of mass and transparency.Nothing to do with radiative fluxes
More atmospheric mass can compensate for a reduction in transparency.
Opacity reduces the amount of conduction that can occur but more mass increases it again.
That solves the conundrum as to why some planets with opaque atmosphere but a high mass have much greater surface temperatures relative to their distance from the sun than does Earth.
Venus and Uranus, due to their opaque atmospheres might have less solar radiation reaching the surface (which reduces conduction and the surface thermal enhancement) but the amount of mass more than compensates so they have much larger surface thermal enhancements than planets like Earth and Mars.
So Steve, does the gaseous composition of a planetary atmosphere play a significant part in the ambient temperature of that atmosphere?
Molecules which have the same energy but are higher up have more PE and therefore less KE. With less KE they must have lower temperature. The Density of the gas forces it upwards due to displacement by colder “heavier” gas, this cools everything by converting heat into height. A very hot planet with weak gravity should end up with a great volume and cool as a result. If a planetary body is left in deep space without sunlight, but a very large mass, the pressure in the centre would be greater than that of the surface. Assuming that energy is distributed evenly throughout, the centre will be hotter than the surface.
It’s fascinating to watch global warming deniers go all-out presenting arguments that are both correct and totally irrelevant.
Yes, the lapse rate is -g/Cp, i.e., it depends only on Cp (heat capacity at constant pressure) and gravity, and not (to first order) on the spectral characteristics of the gases involved.
And yes, it turns out that the dry lapse rates on Earth and Venus are approximately the same (-9.8K/km on earth, -10.4 K/km on Venus) because Venus’s lower gravity is almost canceled out by the lower Cp of its nearly pure CO2 atmosphere.
But all this hardly proves the lack of a greenhouse effect, and here’s why.
Every planet has an effective blackbody temperature that causes it to radiate as much to space as it absorbs from the sun. (We can ignore internally generated heat, which is reasonable for the terrestrial planets.) That effective blackbody temperature depends only on albedo and solar insolation; atmospheric composition is again irrelevant.
But the effective blackbody temperature is usually LOWER than the actual surface temperature of the planet. For example, the earth’s effective blackbody temperature is 255 K (-18C), considerably colder than the actual average surface temperature of about 288 K (+15C). In other words, when you look at the earth from space at long IR wavelengths you are NOT seeing its surface but some level in its atmosphere. And that level depends CRITICALLY on atmospheric composition.
Let’s assume we add more GHG to earth’s atmosphere, increasing its optical depth at its blackbody wavelengths. Even if the lapse rate remains unchanged (as it will if the average Cp is not significantly changed) the altitude of the virtual “longwave IR surface” will increase to keep the effective temperature at 255 K. And guess what? Precisely because the lapse rate is unchanged, the surface will warm up!
So while it’s correct to say that GHGs don’t affect the earth’s lapse rate, it is INCORRECT to claim that doing so won’t warm the surface. It will, and precisely because the lapse rate is unchanged.
“But the effective blackbody temperature is usually LOWER than the actual surface temperature of the planet. For example, the earth’s effective blackbody temperature is 255 K (-18C), considerably colder than the actual average surface temperature of about 288 K (+15C)”
It requires a surface at 288K to radiate to space at 255K simply because the deeper one goes into the mass of an atmosphere the less photonic energy is emitted and the more energy is exchanged in collisional activity instead.
The same parcel of kinetic energy cannot be both radiated to space and passed to adjoining molecules at the same time.
That is why the effective blackbody temperatutre is lower than the actual surface temperature.
The mass and gravity induced lapse rate slope tracks the gradual transition through atmospheric mass from photon emission to collisional exchange.
For Earth that is 33K extra kinetic energy needed at the surface in order to both radiate 255K to space AND hold the mass of the atmosphere in hydrostatic balance against the force of gravity.
If GHGs cause a radiative imbalance then convection neutralises it:
http://www.public.asu.edu/~hhuang38/mae578_lecture_06.pdf
This is simply wrong. As I said, the lapse rate is a function of gravity and heat capacity. Meanwhile, the planet’s effective blackbody temperature is determined by net solar absorption (i.e., total solar insolation and albedo), because everything that is absorbed is re-radiated for there to be equilibrium. If the greenhouse effect were totally absent, and assuming the same albedo and heat capacity, the earth’s surface would cool to the effective blackbody temperature. The whole lapse rate curve would shift to lower temperatures.
Adding a greenhouse gas like CO2, N2O or CH4 (again keeping Cp and albedo constant) has the opposite effect. The optical depth of the atmosphere at long IR increases, the earth’s radiating “surface” moves up, and the surface warms precisely because the lapse rate remains constant.
This is all basic atmospheric physics which I learned in a few hours from a couple of standard textbooks on the subject. If I can learn it, so can you. Then again, I am interested in the actual science. I am not desperately looking to excuse uncontrolled GHG emissions.
The ideal lapse rate set by mass and gravity cannot change otherwise the atmosphere would be lost from either excess heating or excess cooling.
GHGs alter the actual lapse rate but do so in equal and opposite directions in ascending and descending columns of air so that the net thermal effect is zero.
They do accelerate convective overturning but so little that we could never notice compared to natural variability from sun amd oceans.
Regardless of the air’s optical properties, i.e., whether or not it contains any GHGs, there is no convection in a dry atmosphere with an adiabatic lapse rate. That lapse rate is determined by simple energy conservation: the sum of thermal and gravitational potential energy is constant with altitude. A parcel of air at high altitude has greater potential energy than a parcel at low altitude, therefore the high altitude parcel is colder.
Without convection, atmospheric heat can move only by radiation or conduction. Without GHGs, radiation within the atmosphere is also out, leaving only air/ground conduction at the surface. At equilibrium, the temperature of the air at the surface is equal to the surface temperature, so there is no net flow of heat. The surface radiates directly to space and the air does not interfere. The surface therefore achieves whatever temperature is required to balance absorbed solar insolation.
But if the atmosphere does contain GHGs, then parcels of air thermally radiate *and absorb* in all directions. Only the upward radiation at the top of the atmosphere is lost to space. Radiation in other directions is recaptured by other GHG molecules or by the surface, which warms to radiate the extra heat. Back at equilibrium, the sum of thermal and gravitational energy remains constant with altitude but that sum is greater than without GHGs. The lapse rate — the slope of the temperature curve (K/km) — is the same BUT THE ENTIRE CURVE SHIFTS TO THE RIGHT!
Radiation to space no longer occurs at the surface but at some point above it, which assumes the temperature the surface had in the non-GHG case. The greater the GHG concentration, the higher the effective altitude at which the atmosphere radiates to space and the warmer the surface gets because of its greater depth under this effective radiation altitude.
Venus has an atmosphere of roughly 93 bar, with 96.5% CO2. Earth has 1 bar, and .04% CO2. That leaves 1 square meter on Venus with roughly 225,000 times as much CO2 insulating it as a comparable square meter on Earth. The highest value I’ve ever seen for climate sensitivity (on Earth, including every feedback Hansen thought possible) is 6K per doubling of CO2. (Hansen, 2008) To get to Venus’s level of CO2, we’d need to double that in Earth’s atmosphere roughly 18 times. That should give Venus roughly 108K of CO2 induced warming, relative to Earth.
Earth averages about 288K. Ignoring the difference in distance to the sun, which I think we all agree is not a major factor in the temperature difference, that would put Venus at about 396K. Considering I’ve erred on the side of warming for every assumption, (except solar distance) this should be close to an upper limit. Yet the observed surface temperature on Venus is almost twice that. It appears that something other than CO2 is causing the large majority of the temperature difference between the two planets.
Put differently, doubling CO2 on earth is usually assumed to produce an additional radiative forcing of about 3.7W/m^2. Multiplying that by 18 times gives us 66.6W/m^2. Doubling that again, for the difference in solar distance, gives us 133.2W/m^2, or about 10% of the total insolation at Earth’s orbit. Once again, this isn’t enough forcing to get us anywhere close to the observed surface temperature on Venus.
What am I missing?
I don’t think it’s reasonable to take a linear approximation intended for relatively small changes in CO2 on the earth and extrapolate it to the far more extreme conditions on Venus.
“What am I missing?”
Nothing. 🙂
CO2 sensitivity is disproven by your exercise. It must be considerable more than anything suggested to even come close to the Venus surface temperatures
Now if you were to look at the gravity/pressure gradient effect, I bet you would get pretty darn close to the right number.
Oh wait.. that’s already been shown to be the case. 🙂
Did you know that the sunny side and the dark side of Venus have almost the same temperature, despite the long rotation period (over 100 days iirc) and that at the same atmospheric pressure in Venus’s atmosphere as on Earth, it is almost exactly the temperature it should be wrt its distance from the sun.?
Wrong. Go study atmospheric physics.
I wait for you to catch up, in say 10-15 years time.
Actually, you are correct that the temperatures on Venus are nearly uniform, not just between the night and dark sides but also between the poles and equatorial latitudes. That’s because of the very strong thermally-driven circulation in the thick Venusian atmosphere that carries solar heat around the planet.
All that is consistent with established atmospheric physics, the same physics now used to model the sensitivity of the earth’s climate to CO2 levels.
I have a pressure cooker, how does it work?
A pressure cooker doesn’t really have anything to do with a planetary atmosphere, but it works because the boiling point of a liquid like water increases with temperature. So by putting the water under pressure, you can increase the temperature and cook the food faster while keeping the water from boiling away.
“boiling point of a liquid like water increases with temperature.”
you meant… “boiling point of a liquid like water increases with pressure”.
Correct. My brain must not have been engaged when I wrote that.