How Similar is Water On Mars To Water On Earth?
New evidence suggesting the existence of water flowing on Mars indicates the possibility for life to exist on the Red Planet.
NASA’s Mars Reconnaissance Orbiter have taken images and videos of dark, finger-like features running down a number of Martian slopes in the middle latitudes of the Planet’s southern hemisphere. The lines become more visible in the warm season, suggesting that they could have been created by the flow of certain volatile chemicals which boils at low temperatures.
These flows then dry up in colder seasons. The strongest candidates are water and carbon dioxide, according to a study by planetary geologists at the University of Arizona.
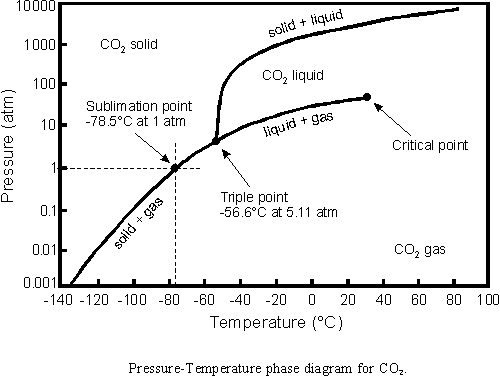
Atmospheric pressure on Mars (or Earth) is much too low to have liquid CO2. What are they talking about?
I’m pretty sure too that H2O is H2O, whether it is on Mars or Earth.


Great rocket scientists we have these days.
That graph looks like old style physics to me – not a computer model. You really need a computer model if you want to study other scenarios.
I remember studying triple points in second year physical chemistry. These are supposed to be planetary geologists and they are talking about liquid CO2 on Mars? This is basic material!
oooooookaaaaaaaaayy…………………………………..
My God. This is 7th grade physical science level stuff. Their heads must be full of CO2.
LOL
Even better:
Because everyone knows that subliming CO2 frost creates downward flows . . .
It’s perfectly possible for liquid CO2 to exist on Mars if it’s underground – the pressure from the overlying rock brings it into the correct part of the phase diagram. It could potentially burst to the surface and boil off, creating some flow features in the process, much like a geyser in Yellowstone. There’s some circumstantial evidence this may be what’s going on – many of the flow features are in the Southern highlands where liquid water is even less possible than liquid CO2. Moreover, the features often start ~100m from the top of a cliff, which is just the right zone for liquid CO2.
http://www.unisci.com/stories/20012/0402013.htm
I see. So that is underground liquid CO2 running across the surface in a nice linear streamflow pattern.
Have you seen the animation I posted? The water moves slowly across the surface. There is no steam.
Nope, can’t locate any animation posted in the last 3 pages of posts. If you’re talking about the one in this BBC report (i.e. the one from the latest Science paper), then that’s from a completely different part of Mars. Not all Martian erosional features have to have the same cause!
http://www.bbc.co.uk/news/science-environment-14408928
In fact, there’s at least three things getting mixed up here:
1) Various dendritic drainage gullies, salt pans etc. These are very good evidence for past water on Mars, but don’t tell us about the present day. Large areas of open water are not stable anywhere on Mars under current conditions.
2) Drainage patterns observed in the process of formation in the Southern Highlands. These are in an area where liquid water isn’t possible, but liquid CO2 (which immediately turns into slurry when it erupts) is feasible.
3) Drainage patterns observed in the process of formation on sunward crater rims during summer. This is the latest piece of evidence, and seems most likely to be due to liquid water.
A note about (2) – the Southern highlands have massive deposits of frozen CO2 ice – thousands of cubic kilometers of it. The erosional features referred to seem to be coming from these – when the deposits begin to warn in the summer, the top layer obviously sublimes, but you also get a melt zone some hundreds of meters below the surface. The liquid CO2 can erupt if a weakness in the overlying layers of dry ice + sand allow it to be forced to the surface. The reservoir is replenished by CO2 freezing out during the winter.
Get a block of dry ice and set it on the table. Watch what happens.
Wow, the BBC. I’ve never heard of such good science.
Regardless of how it comes out of the rock stratum, how does it get back in there every year? Assuming it is CO2, obviously. I mean, how on Barsoom does CO2 enter a highly pressurized rock stratum, and then somehow become compressed, and then exude? Does CO2 expand when frozen, like water, and thus increase in pressure as it sublimes from the atmosphere into the strata?
If you expose a block of dry ice to the atmosphere, it forms steam. It never goes to a liquid stage.
Well, yes, but even assuming the silly notion that liquid CO2 flowing under highly pressurized rock strata is, in some weird universe, actually happening*, how would it get back in there to flow next year? Or do they presume some massive reservoir? A massive reservoir that somehow isn’t depressurized, and showing signs of collapse on the overlying rock?
Or is it that Global Warming on Earth is uprooting the very laws of physics? It’s Worse Than We Thought!®
*yeah, the layer of sand that shows signs of liquid flow is a really highly pressurized rock stratum, I get that. Awesome science there, kiddos.
Good point Steve. How will they explain this away now?
Steve,
good to see you have learned about triple points. I just googled this and some interesting material came up that I was not aware of.
http://wattsupwiththat.com/2010/08/29/sea-ice-news-20/
My favorite quote from that interesting exchange is this “As I stated in my last post, it is impossible to create a perfect triple point equilibrium condition, so we scientists deal with the real world instead.” The real world apparently having the arctic with almost no atmospheric pressure.
And here I thought that Steve had made the craziest denials of reality in his arguments with me. I stand in awe of what happened on Anthony’s site.
next to that fiasco Hansen might as well have said Manhattan would be underwater by 2008!
You are a moron dedicated to wasting everyone’s time.
Reading comprehension strikes again! Good try, “Tony Duncan”, but I hope everyone who sees your comment notes that the quoted exchange says precisely the opposite of what you’ve tried to claim here.
Tony,
Feel free to go to WUWT and post exactly what I said that you disagree with. If you keep spamming bullshit urban legend straw men, you will be spam.
Let Tony stay. He’s entertainment!
Tony, if you’d bother to research this properly, you’d see that this has been covered more than adequately. Heck, you don’t even have to try, just look at the first few sentences of the triple point article on Wikipedia:
[emphasis added]
Skip a sentence and then there’s this:
What do you think the partial vapor pressure of water is immediately above an ocean? Seriously, I lost a massive amount of respect for so many commenters in the blogosphere on this topic because this is very basic Physical Chemistry or Chemical Engineering Thermo material. BASIC. The actual world out there is so much more complicated it’s not funny, and yet people don’t even understand what partial or vapor pressures are. When I tried to send CTM info showing Steve was correct, his answer was some sort of bizzaro thing that I don’t remember but clearly confused partial/vapor pressure and had the value all wrong too. Now, don’t go thinking that I’m one of those people that agrees with Steve all the time b/c people that have been here for a while know I disagree with him on plenty of things.
The discussion Tony mentions has been nitpicked in detail. A good place to start would be to read this for an idea of where it ended (comments section):
http://stevengoddard.wordpress.com/2010/09/02/a-banner-day-for-climate-censorship/
Basically, my opinions are that the triple point comment was unneeded in the first place, people jumped all over it, but they were just nitpicking because had it read “a triple point” or “the triple point at 1 atm” it would have been 100% right…but he said “the triple point” which is a bit ambiguous. In other words, it’s a whole lot of argument about nothing and is all about oneupmanship.
-Scott
Scott,
Scott
I am certainly willing to understand this better , but as I said I just ran into this googling triple point.
I am NOT a scientist, and it seemed clear to me that triple point means THE point where a material can be in all three states EQUALLY, and that Steve refused to acknowledge the huge difference between that and water being at 0°C (or -1.9) where it can be equally either liquid or solid but NOT steam. The fact that Anthony shut his comments down seemed to indicate that even he thought Steve’s comments were ridiculous.
What you seem to be saying indicates to me that the triple point then becomes an almost worthless concept in physics. Again, I am not a scientist and may be completely wrong about this, but I don’t believe that ANYWHERE on the surface of the earth at any temperature or pressure there exists a situation where H2O can be liquid, gas, or solid without a strong tendency to only one or two of those states at any one time. Certainly in real world situations there are many factors that can change the likelihood of any of the three states. Humidity, wind, contaminants, etc, but I just don’t see anyway to relate those factors to the phrase “triple point”. The fact that ice can sublimate and water evaporate at temperatures below the normal phase transition point seems quite ludicrous if connecting it to “triple point”. Do you know of any scientist who has used the term in discussing H2O in the arctic”? a quick google search revealed nothing for me.
I will read the post you linked to and I am sure I will learn a lot more about the subject. Which I state again is one of the great things about this site. I admit up front I could be completely wrong, and if so it will certainly change my (very slight) understanding of physical chemistry.
Your mind is in three states equally. Disordered, severely disordered, and extremely disordered.
Having liquid, solid and gaseous water in the same place at the same time is the norm on Earth.
http://www.hotelarctic.com/om_hotel_arctic/webcam/
http://www.youtube.com/watch?v=WAOxY_nHdew
Steve,
I am sorry, WHERE did I say anything that remotely contradicts this statement?
“Having liquid, solid and gaseous water in the same place at the same time is the norm on Earth.”
You really do need to read the links you post and your responses to comments.
Oh, I get it, You have discovered that the TRIPLE point is the norm on everywhere on Earth. As I always suggest. Publish this discovery and you will be famous!
(hoping that Scott actually answers my comment, so that there can be a rational conversation)
You are constantly changing the subject and raising straw man arguments. I can scarcely remember a time when you were on topic. There isn’t any liquid CO2 on Mars.
Steve,
I certainly did change the subject on this. I was just so taken by that discovery about the triple point thing.
As for liquid CO2 on mars, it seems that you graph is not a fully valuable resource for the complex reality of CO2 on Mars. Apparently scientists who propose this possibility are aware of the triple point of CO2, and explain it clearly.
http://www.unisci.com/stories/20012/0402013.htm
Ah. The triple point. Where ice, liquid water, and water vapor all coexist. Unheard of on Earth.
Hi Tony,
I’m a bit in a hurry, but I’ll try to answer your inquiries concisely yet at least somewhat thoroughly.
First off, the concept of all three existing at once is a bit of a straw man (and you aren’t the one who brought it up, I know). It’s easy to throw ice into a glass of water and say all three are present. But they aren’t in equilibrium there. So when anyone tries to use that argument, put them in their place.
Getting more to the heart of the matter:
First, the ideal triple point as you’re trying to argue towards is only applicable in theory or very nearly in a controlled lab. It requires the pure compound in a closed system. But this does not make the idea worthless. This is because it’s much more applicable by substituting partial pressure for total pressure, allowing one to work in the real atmosphere. Now, technically the phase diagram might change a bit due to nonideal interactions and dissolution of some of the other gases into the liquid and solid phases. But those are minor changes, and with our atmosphere of 1 atm pressure and the relatively ideal behaviors of oxygen, nitrogen, and argon at those pressures, it works pretty well. Thus, substituting the partial pressure of water for the total pressure works very well and is good enough for almost any application we care about. Concerning your “anywhere” comment, my response to that is that anywhere on the planet where the dew point and temperature are both 0 C will be a triple point of water. Why? Because the air is saturated with all the water vapor it can hold while the condensed phases (solid and liquid) are also in equilibrium. Thus, no more solid/liquid will be volatilizing and all three are present and stable. As to what constitutes “close” to this is obviously subjective, though I would argue that the Arctic conditions have to be close…the temperature is clearly near 0 C, and the air is likely near saturation (note that this is a good point…people keep arguing that the pressure is too high for a triple point, but it’s not at all…the partial pressure is too LOW).
Now if you don’t know much chemistry wrt partial vs vapor pressure, I’ll give a quick explanation. The partial pressure is the pressure exerted by only a specific gas. Take air at a total pressure of 1 atm (assume composition is N2=78%, O2=21%, Ar=1%). The partial pressure of nitrogen = 0.78 atm, oxygen = 0.21 atm, and argon = 0.01 atm. Now, here at 5000 ft our pressure is ~0.84 atm IIRC. Thus, the partial pressures are more like nitrogen=0.65 atm, oxygen=0.18 atm, and argon = 0.008 atm.
So what’s vapor pressure? That’s the equilibrium partial pressure of the gas phase substance above the condensed phase. It’s very dependent on temperature. Once the partial pressure of the vapor above the condensed equals the vapor pressure, no more evaporation (or sublimation if the solid phase) takes place. With that knowledge, you should be able to tell me what the vapor pressure of water is at 100 C (it’s 1 atm!) Also, if I boil water here at 5000 ft, I’ll find that it boils several degrees below 100 C because boiling occurs once the vapor pressure reaches the total pressure, which is ~0.84 atm. (Note that for many compounds you can get a good estimate of the vapor pressure of a substance as a function of temperature using the Antoine Equation).
Now, with those two concepts covered, we can quickly understand dew point and relative humidity. Relative humidity is simply the partial pressure of water divided by the vapor pressure. Thus, once it’s 100%, no more evaporation. The dew point represents the temperature where the vapor pressure (which is dependent on the temperature) equals the current partial pressure of water. So say you had a hot container of water vapor at 1 atm…its dew point would be 100 C.
Okay, I think that about covers it, let me know if it’s not clear.
—
So what does the triple point have to do with the Arctic melt? IMO, not much. It’s more important that the conditions are near the melting point of water (which over the Arctic ice in the summer should be near the triple point). But who really cares about the gas phase? The amount of sublimation/deposition of water vapor wrt the ice is minimal…in the link I provided you can see where a couple of us estimated it and found it to be trivial (on the order of mm at most…which is probably an overestimate).
What bothers me about this subject is how it blew up so much and no one involved was able to make both a clear argument and get it right…
-Scott
What bothers me about this subject is how it blew up so much and no one involved was able to make both a clear argument and get it right…
==============================================
Scott, it was a perfect storm…. styles and insecurity. On this subject, you actually took the time to explain…. for some, it may be a bit remedial, but for others, it is illuminating. You were able to clear up the confusion because 1) you know what the heck you’re talking about. and 2) you actually took the time. You could do this because you’re well versed in the subject, so you don’t have fear of appearing ignorant or being challenged. In the discussions of the past, the others could not do what you have just done because while they knew what they were saying, they didn’t understand how these things apply. The “pressure” thing really tripped a lot of people.
They couldn’t do more to rebut Steve, because while they knew statements of “triple point”, there was no understanding. Fear of ridicule and appearing ignorant on the subject prevented going to any depth on the subject. Sadly, on the prior discussions, learning and understanding took a hit.
Well done.
James
I imagine you hit the target pretty well James.
The whole thing left a bad taste in my mouth because I realized how ignorant people on both sides of the discussion were and how it was more about egos and such. In this case, I could tell because this is a topic I’m well-versed on and use routinely (though it wouldn’t surprise me if a hardcore, old-school PChem expert could show how I was a little off on some of my interpretation of the material). However, many of the people involved (and who got it wrong) were people I listened to on other things in which I was NOT well versed and took their message hook, line, and sinker. I guess that’s called naivety. Anyway, it really got me to questioning what people in the blogosphere say more than I used to. I’d already gotten to the point of questioning what scientists in the lab said because of many bad experiences in graduate school with Ph.D. candidates (and postdocs…and professors really too) who were completely full of BS.
I guess it’s just human to be overly defensive, aggressive, and boastful…particularly when one is insecure about a topic. I’ve done the same thing before so I shouldn’t be pointing too many fingers…
-Scott
Scott,
thanks for the explanation. It actually was at just about the right level for me. It still strikes me that using the term triple point in the way Steve said makes no sense whatsoever. What you are talking about is the freezing point of H2O. I think I understand that the temperature and partial pressure of H2O and other factors affect evaporation but again “triple point” seems like a very specific situation where solid, liquid, and gas, are equally likely.
In the article I just read about mars and the possibility of liquid CO2 having a role in the formation of gullys in the south there is no mention of triple points, just freezing melting, explosive evaporation, and condensation. Can you point me to any place where scientists use the term “triple point” when referring to the melting point of gases?
as for why the situation blew up, it seems pretty clear that it was because Steve was unwilling to back down and explain that what he meant was not exactly what he had written. As you can see from his responses to me on just this post, he purposefully misreads what I have written and makes sarcastic comments that are totally irrelevant to anything I am talking about. If he had bothered to do what you have just done, none of that would have happened. As you write exactly to the point of why my initial reaction to reading that thread was incredulity, the “sublimation, deposition, of water vapor” is negligible, which even someone like me knows, so what is the possible point of using the term triple point.
It makes no sense because you an idiot. Water coexists in all three phases at the same location on Earth.
Steve,
see what I mean?
Yes, I see that you are an idiot and always trying to raise straw man arguments. There is no liquid CO2 on Mars
Wow, missed this post while away over the weekend. This thread actually made me laugh out loud several times.
Well, Tony, that one really takes the cake. Maybe you can point to where anyone besides you mentions the term “triple point” when referring to the melting point of gases.
Perhaps I can give you a few starting points: Steven Goddard mentions the triple point of CO2 which is a(n):
a) gas
b) liquid
c) solid
d) chemical substance
e) unloved son
Below the triple point of a chemical substance, what state(s) can that chemical substance not exist in?
a) solid
b) liquid
c) gas
d) balloon animals
e) chemical substance again?
When discussing the triple point of Carbon dioxide, what does Tony Duncan prefer to talk about?
a) Water
b) Anthony Watts
c) sword swalloing
d) the melting point(s) of gas(es)
e) his mother never loved him
Stark,
Don’t you know anything about physics? the melting point of a gas is when it goes into a plasma state.. this is important in order to understand the longer term effects of global warming. At 3-4°C increase/ century the Earth’s temp will be high enough in less than 100,000 years that those kind of calculations will be necessary, and by then all you deniers will have to eat crow, let me tell you!
PS. How did you know I do sword-swallowing?
Getting back to rain on Mars… I’ve spent the last year of my spare time working on a video that uses a revised version of Relativity to explain rain on Mars among other things. I finished last night. It’s radical, so please keep an open mind. Would appreciate feedback on it. http://www.youtube.com/watch?v=J6kcdFGa6tI
Thanks;
Larry [email protected]