http://www.globalwarmingart.com/images/7/7c/Atmospheric_Transmission.png
Using the RRTM radiative transfer model (incorporated in NCAR’s climate and weather models) we can easily calculate that a 100% CO2 atmosphere would be extremely cold.
CO2 only absorbs a few narrow bands of LW radiation, meaning the vast majority would radiate directly out into space.

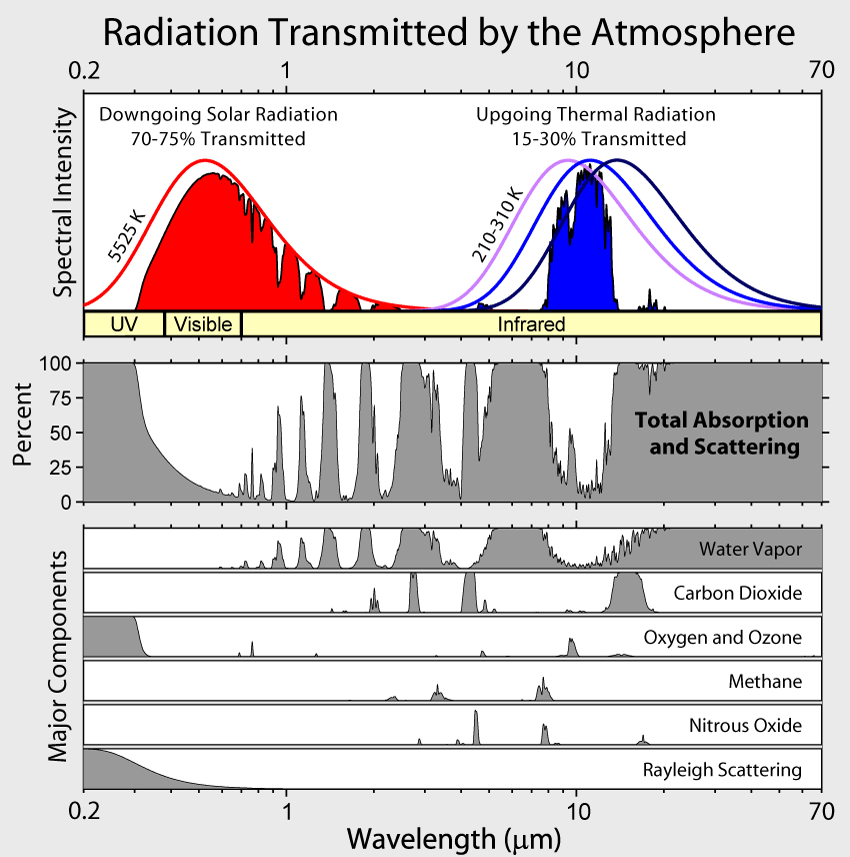

How does Venus fit into this picture?
Venus is hot because it has a very thick atmosphere. The lapse rate on Venus is similar to Earth. Mars atmosphere has about the same composition as Venus and is extremely cold.
Venusians don’t use twisty lightbulbs.
I think even Brendan might agree with me on this.
As Steven states, the atmospheric pressure is one driver of Venus’s climate.
Venus is also hot because its 40% closer to the sun, so the incoming radiation is nearly double.
For what it is worth, when you look at a temperature profile of Venus’s atmosphere, at about 30 km (1 bar pressure), the temperature is warmer. Calculate out the near doubling of w/m2 from solar proximity, and its a few degrees K warmer than earth.
No, The atmospheric albedo of Venus is much higher than Earth (due to clouds.) Venus surface receives less SW radiation than Earth’s surface.
Venus’s surface receives less radiation, but the atmosphere receives more, especially at the high altitude (3o km) I was referring to.
Just a thought, but with the high Albedo lower down, the upper atmosphere would be subject to this radiation twice. Once incoming, then outgoing.
I also recall that there was some speculation that Venus may have a greenhouse effect from sulfur compounds, at this high altitude, instead of a negative forcing.
Let me see if I can find that reference.
Most of the sunlight received at Venus TOA is reflected. That is why the planet is so bright.
Venus would only be 70 degrees celcius if it had the same atmosphere as earth. It’s 400 degrees.
The atmospheric pressure on Venus 100X higher than Earth. It has nothing to do with the chemical composition.
Tell GLazed (doughnut) that.
Yeppers, now here’s the question. When CO2 or any other gas is emitted into the atmosphere, does the gas displace other gases or does it simply add to the composition of the atmosphere?
Simple example:
CH4 + 2O2 –> CO2 + 2H2O
So in this case 2 oxygen molecules are exchanged for a CO2 and 2 waters. So we added to the composition.
my mistake. one bar of pressure on Venus is about 50 km, not 30.
Good post Steve; wish I’d thought of it. Does that radiative transfer model run under Linux?
Did you extract a temperature value (other than “frozen”) from the model? I’ve been engaged in discussion with some polite and knowledgeable folk at
http://wonkroom.thinkprogress.org/2010/10/24/science-v-buck/
Apparently Ken Buck expressed his disbelief in the global warming thing a few days ago, and Climate Scientists named Denning, Ojima, and Ammann jumped in to correct his ignorance. Not asking you to do my work for me; just giving you the background.
Hope this post will be around for a few days; I referred readers of that post here, to see just how poor a GHG CO2 is.
Thnx
rcs
It does, but requires a proprietary compiler. You can get a 30 day evaluation license from PGI.
Thank you Mr. Savage.
I enjoyed reading your comments there.
A new perspective for me as well. Water means everything. Even if CO2 were capable of capturing and transferring additional energy to water vapor, it seems existing clouds would simply evaporate and rise…. only to condense again at a higher altitude. When enough water vapor coalesces into droplets, they’re too heavy to remain buoyant, and fall back toward earth.
(It seems) the energy dissipation process of the hydrologic cycle wouldn’t change at all, but would simply occur at a higher altitude.
Almost all LW infra-red radiation escaping to space is emitted by GHGs. Without GHGs the atmosphere would receive a small but steady warming by conduction/convection from the surface, with additional small amounts absorbed from incoming solar and outgoing surface radiation. With little radiative capability, the nitrogen/oxygen atmosphere would thus warm, eventually exceeding the current temperature (a runaway non-Greenhouse effect?).
Since it’s GHGs which radiate heat away from the atmosphere, the resulting temperature is lower, and just like Goldilocks’ porridge is “just right” for life on Earth.
Increasing the level of any GHG will result (omitting any saturation/masking effect) in that gas absorbing and therefore radiating more – it’s the basis of the fallacious “Greenhouse Effect”. If there’s more radiation, this must include radiation to space, yet we’re supposed to believe that an increase in GHGs will result in a reduction in outgoing radiation. It doesn’t take a PhD in physics to see the flaw in THAT argument. In the upside-down-world of the climate scientist and modeller, it seems that a hotter Earth would radiate less than a cooler Earth.
The long-dead pioneers of physics must be turning in their graves.
Question: If Earth had an atmosphere of 100% carbon dioxide and no oceans (to transmit water vapor to the atmosphere), how many bars (pascals) of atmospheric pressure (at the current sea level of altitude) of CO2 would be necessary to achieve the same current global mean atmospheric temperature (approx. 14-15 degrees C.)based on the IR absorption properties of CO2 and the mean annual solar radiance received by the Earth? Without being a physicist, I sense that the atmospheric pressure of CO2 would have to be substantially greater than the current atmospheric pressure to achieve the same mean global temperature in the absence of water vapor?