The climate doesn’t care about human neuroses or political agendas. Corals evolved with CO2 levels above 4,000 PPM CO2.
400 PPM CO2 means nothing – other than another meaningless number for clueless people to get hysterical about. CO2 is at historically low levels in the geological record.


In my area I have measured CO2 levels in the 700 ppm range. The only affect I noticed is that my outdoor potted plants grow really, really well.
http://www.skepticalscience.com/co2-higher-in-past.htm
From your link, GT – “As our paleo techniques improve and other discoveries emerge this story will no doubt be refined.”
Story is the operative word. CAGW is a wonderful story that has now been shown to be almost completely erroneous and was really the PR cover story for a Carbon Credit scam of biblical proportions. But hey, “shit happens!”
I’m assuming that’s why you brought it to our attention.
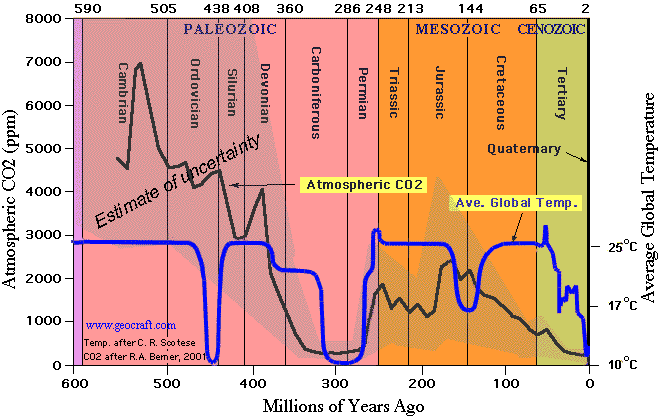
Cook talks about the Ordovician while ignoring the second half of the Cretaceous in his 2010 post. See the graphic above. He claims that “CO2 is the greatest forcing and also the fastest rising.” That must explain the temperature standstill then. 😉
I love how a small group of global warming hacks believe that they are entitled to rewrite science and history based on their current drug induced mental state.
From the same site as you got the graphic from is an excellent write-up on American coal reserves…
http://www.geocraft.com/WVFossils/Energy.html
How dare we exploit our own abundant resources, lower the cost of energy and reduce our dependence on energy from unstable parts of the world. Those people must be crazy! (sarc!)
Well, it’s not like you can use it for anything else but burn it. Is there?
/sarcoff
An old use of coal used to be for putting it in certain kid’s stockings at Christmas…
If we brought that habit back, the way kids behave today, we might run out of supply.
I was going to ask a question
Here is the answer
http://en.wikipedia.org/wiki/Ordovician–Silurian_extinction_event
http://en.wikipedia.org/wiki/Carboniferous
Apparently the two zero-co2 periods coincide with extinction events.
Makes you feel all warm and fuzzy about the CO2.
Yep, 300+ million years of life wiped away. How long has modern man been on the planet?
I recently read on Skeptical Science, some climate scientist saying we need to “”get atmospheric CO2 down to zero to save the planet.”
They really haven’t thought that argument through, have they?
The intelligent HOPE that CO2 goes to at-least 400 ppm or higher:
http://www.youtube.com/watch?v=P2qVNK6zFgE
Fine video, thanks Paul V. Sheridan and CO2Science
The knowledgeable know that CO2 is but one factor that determines the rate of plant growth and that if climate change as a result of increased CO2 causes other factors, such as temperature and soil moisture, to change in a deleterious way then the overall effect can be a decline in growth.
goldilocks
Hey Slioch! I conducted my first CO2 soybean plant experiment in 1977, and the results have never changed. All things being equal, the more CO2 a soybean plants receives, the larger and healthier it grows, and the less water it needs as related to its biomass.
We need more CO2, not less. Plants today are starved shadows of their predecessors.
Slioch is the new T.O.O. The language is the same. Me would love to chat I’m sure.
I think the concern is that even though corals evolved during a period of high CO2 they have since adapted to a regime of low CO2. So the question is, can they adapt quickly enough if CO2 increases as rapidly as it has been doing now? I suspect the answer is ‘yes’ but before anyone declares that corals are all going to die and/or coral will do fine, some actual research would be useful. Call me old fashioned…
There isn’t any reason to believe that corals are affected by minor variations in CO2.
The fear is that because corals build their shells from calcium carbonate, a somewhat more acidic ocean would make this task more difficult for the organism. The question is, does it in practice?
All shells are built from calcium carbonate, and always have been. The oceans are buffered by huge amounts of CaCO3 (limestone) which keeps the pH from varying outside of a very narrow range,.
People promoting ocean acidification are mental midgets.
Will, corals use their own CO2 to dissolve calcium carbonate first, so they can lay it back down as their matrix….
…a lower ambient pH just makes that easier for them
Will, I propagate ‘stony’ corals … they don’t mind a bit of alkalinity but at the same time I’ve had my system pH as low as 7.6 and have seen no deleterious effect on my stock. The absurd idea that ocean pH can become acidic is fairytale stuff … the ocean is so heavily buffered with carbonates that acidification is not practicably possible. I had this chat with a (catastrophic) marine biologist not long ago and was astounded by his ignorance of atmospheric physics and the general circulation systems, ocean and air … and I’m no expert, just educated to think.
Street, check the pH of their bag water the next time you ship one….
….it should have dissolved
STeve: Do you have a source for the limestone levels? I get sick and tired of hearing the “ocean acidification” argument and would love to put some numbers together to demonstrate just how insane they are.
Mike, it can only be created in the lab…..you have to run enough CO2 to completely deplete the buffer first
Steve Goddard stated, “the oceans are buffered by huge amounts of CaCO3 (limestone)”
That, actually, is the problem, because the buffering reaction is:
CaCO3(s) + CO2 + H2O ==> 2Ca(HCO3)(aq)
In other words, the buffer works by dissolving solid calcium carbonate.
Which, of course, is what shells are made from.
You are a moron. That equation is for water which is saturated with CO2.
Using your brilliant thought process, there would be no limestone.
Why is it that lefties imagine that any stupid idea which pops into their head must be true?
Just trying to help Slioch understand oceanic chemistry:
CO2 (g) CO2 (aq)
CO2 (aq) +H2O H2CO3 (aq)
H2CO3 (aq) H+(aq) + HCO3- (aq)
Evolutionary biology allows ocean organisms to secrete Calcium Carbonate skeletons, shells and corals. This is facilitated through turning Ca into CaCO3.
Ca++(aq) + 2HCO3-(aq) CaCO3 (s) +CO2 +H2O
Without dissolved CO2 there would be no new CaCO3 to accumulate, with pH one contributing factor in which way the reaction goes. Alkaline pH facilitates the reaction to produce CaCO3. Gee whiz, Slioch! Without dissolved CO2, there would be no coral reefs, fish or oceanic life!! Who knew?
Most of this chemistry happened over millenia, particularly 600-300 MYA. If the pH of the ocean ever dropped below 7, then sure watch out. But you see Steven is correct. The ocean is buffered by bajillions of tonnes of CaCO3 (aq) that the global oceanic pH will not be changing for who knows how long, if ever. And ancient limestone deposits are washing into the ocean all the time. Any geologist could tell you that. But what would someone qualified in geological science know about oceanic chemistry….
http://www.coralscience.org/main/articles/biochemistry-2/how-reefs-grow
Lost all the equal signs but you get the drift.
Steve Goddard appears unable to discourse with someone who disagrees with his statements without flinging insults around, whilst thinking that he can divine a person’s political leanings from his expression of chemical equations, which is rather strange.
As to the small amount of substance in Steve’s reply:
1. “That equation is for water which is saturated with CO2.” No, it is not. The equilibrium amount of CO2 in a saturated solution depends upon the partial pressure of the CO2 in the atmosphere above the water and upon the temperature of the water. With a small partial pressure, the amount of CO2 in a saturated solution would be unable to dissolve any CaCO3(s), with a large partial pressure that reaction would occur. In other words, whether or not the solution is saturated is irrelevant.
2. Quite how Steve works out that if any CaCO3(s) were to dissolve then “there would be no limestone” I shudder to think. Perhaps it would be best just to ignore that slip.
Andy Oz does at least address the chemistry, but he confuses limestone (which is to what Steve referred and I answered) with CaCO3(aq), to which I did not refer.
It is indeed the case that the carbonate ion (CO3–(aq)) is the main buffer against increased acidity by the addition of CO2 to seawater, via the reaction:
CO3–(aq) + CO2 + H2O ==> 2HCO3-
but that was not to what Steve referred.
As to you statement, “Without dissolved CO2 there would be no new CaCO3 to accumulate”, that is, of course, correct, but that does not negate that fact that with high concentrations of CO2 that CaCO3(s) will dissolve again, via the reaction I gave previously.
Dear Slioch,
The CaCO3 I referred to is solid, that’s what the CaCO3 (s) stands for i.e. Limestone.
At what pH does Limestone dissolve? Unless the ocean pH is less than 7, then the reaction you describe cannot happen. It is a buffered solution as described by Steven.
Why argue with a fact unless it doesn’t fit your hypothesis?
You don’t understand the first thing about carbonate chemistry, so you went to Wikipedia and cut and pasted a formula without the line above it that read
You are both ignorant and dishonest. That is not civil.
Andy Oz
No, you did not refer to CaCO3(s) (or limestone as Steve had done) with respect to buffering, you referred to CaCO3(aq) (you should have referred to CO3–, but that is a minor point).
Nor is it true to claim that solution of CaCO3(s) cannot happen unless the ocean pH is less than 7: that is simplistic, there is not a simple cut-off such you imply.
There is a series of equilibrium reactions, each with its temperature dependent equilibrium constant, all of which have to be mutually satisfied at equilibrium.
For example the equilibrium constant (solubility product) of CaCO3(s) Ca(aq) + CO3–(aq) is about 5×10^-9 @25C, which is low, but not zero (and, incidentally, shows that as [CO3–] (carbonate concentration) is reduced, as happens as CO2 is increased, then CaCO3(s) become more soluble.)
You have no idea what you are talking about. Limestones form because there is CO2 in the atmosphere, which dissolves in sea water and precipitates out as limestone.
You should learn the fundamentals of carbonate chemistry before you start cutting and pasting formulas you don’t understand.
Tried to help you out Slioch, but I guess I’m wasting my time. Even gave you a link that details oceanic chemistry and the creation of limestone. Steve’s right, you’re wrong.
It’s a bit like trying to show a color blind person what’s red and whats green.
If you can’t see it, I’ll accept you have an impairment and keep moving.
stevengoddard April 30, 2013 at 10:49 am
I don’t think any impartial onlooker would have any difficulty in deciding which of the two of us is being uncivil.
As for cutting and pasting from Wiki, you, by your own admission, have done that. I have not.
If you don’t understand the series of equilibria involved in carbonate chemistry, that is fine – it is a complex subject – but you do yourself no favours by intemperate responses to someone who is attempting to explain some of it.
As for your pasting of the statement, “Calcium carbonate will react with water that is saturated with carbon dioxide to form the soluble calcium bicarbonate”. That is a simplistic, incomplete and misleading statement. Yes, calcium carbonate will react with water saturated with CO2, but it will also react with water in which the CO2 is unsaturated.
The reaction in question is more correctly written as an equilibrium reaction:
CaCO3(s) + H2CO3 2Ca(HCO3)(aq) (note the double arrow)
for which the equilibrium constant is thus:
[Ca++][HCO3 -]^2/[H2CO3] = 4.4 x 10^-5 (yes I did have to look that number up, but at least I understand what it means). (The concentration of water and CaCO3(s) are constants and don’t appear in the equilibrium constant)
Basically what that equilibrium constant is telling you is that ANY increase in concentration of CO2 will cause solution of CaCO3(s).
Claiming that CaCO3(s) will only dissolve when “CO2 is saturated” (Steve) or “pH is less than seven” (Andy) are both wrong, it is as simple as that.
I had forgotten that double arrows get weeded out in the formatting. There should be one in the above equation.
Andy Oz, April 30, 2013 at 12:00 pm, said he gave me “a link that details oceanic chemistry and the creation of limestone.”
Well, no, actually the article mainly details the biochemical processes involved in producing CaCO3(s) in coral formation.
But it does state,
“The current rise in atmospheric CO2-concentration causes oceanic pH levels to drop slowly, as they absorb about 20% of this greenhouse gas. When CO2 dissolves in water, it lowers the pH value by releasing H+-ions. If current CO2-emissions persist, this level will drop to about 7.4 in the year 2150, dissolving entire coral reefs. Long before that, bleaching will have devastated virtually all reefs leaving behind barren patches of rubble. Even now, a decline in coral calcification is noticeable”
Which, is basically summarising the dangers of human CO2 emissions, as I have briefly discussed.
Complete bullshit. Most reefs formed with CO2 much higher. There is no shortage of completely incompetent scientists publishing papers.
600 millions years ago… …are you serious???
My greatest fear is that I will have to mow my lawn more often.
“CO2 is at historically low levels”.
In human historical terms that is not true.
The first time in the whole of human history that atmospheric CO2 concentration exceeded 300ppmv was around 1910.
CO2 is now approaching 400ppmv, a level not exceeded for around 15,000,000 years.
Quote from Aradhna Tripati, a Univ. California, LA,
assistant professor in the department of Earth and space sciences and
the department of atmospheric and oceanic sciences: “The last time
carbon dioxide levels were apparently as high as they are today — and
were sustained at those levels — global temperatures were 5 to 10
degrees Fahrenheit higher than they are today, the sea level was
approximately 75 to 120 feet higher than today, there was no permanent
sea ice cap in the Arctic and very little ice on Antarctica and
Greenland,”
And there was an ice age during the Ordovician with CO2 at 4,000 PPM.
“4000ppm? That is by no means known to be the case.
Glaciations are influenced by the distribution of the continents and the strength of solar radiation, both of which were radically different in Ordovician times, so direct comparisons with the present situation is unsafe. Further, that figure of 4000ppm has been challenged by more recent research that finds evidence of a decrease in CO2 (possibly caused by weathering of basaltic lava flows) at that time. Richard Alley, for example, has dismissed the “glaciation with 4,000ppm in the Ordovician meme”, for example, here: http://vimeo.com/34099316 at the 2009 AGU meeting.
The bottom line is that we have far a better understanding of events 15,000,000 years ago than during the Ordovician and both the continents and the sun were similar then to the present, so a comparison with today is far more robust. And that time tells us that CO2 held at 400ppmv leads to temperatures 5 to 10 degF higher and sea level approximately 75 to 120 feet higher than today.
Homer. In case you haven’t noticed, most of the earth’s land is currently located near the poles – like during the Ordovician when CO2 was much higher.
“The pre-industrial CO2 level was not significantly lower than current levels. Neither they nor the present readings are high relatively to the geologic record. The entire output of computer climate models begins with the assumption that pre- industrial levels were measurably lower. Elimination of this assumption further undermines the claim that the warming in the industrial era period was due to human addition of CO2 to the atmosphere. Combined with their assumption that CO2 causes temperature increase when all records show the opposite then it is not surprising that IPCC predictions of temperature increase are consistently wrong.”
http://www.friendsofscience.org/assets/documents/FoS%20Pre-industrial%20CO2.pdf
We have a lot to learn about CO2 levels before we start making claims.
All stories have to have a foundation and we look no further than the IPCC for the starting point on acid oceans: Climate Change 2007: Working Group I: The Physical Science Basis, 5.4.2.3 Ocean Acidification by Carbon Dioxide.
(Acid Seas – Back To Basic, http://scienceandpublicpolicy.org/originals/acid_seas.html)
“The uptake of anthropogenic carbon by the ocean changes the chemical equilibrium of the ocean. Dissolved CO2 forms a weak acid. As CO2 increases, pH decreases, that is, the ocean becomes more acidic. Ocean pH can be computed from measurements of dissolved inorganic carbon (DIC) and alkalinity. A decrease in surface pH of 0.1 over the global ocean was calculated from the estimated uptake of anthropogenic carbon between 1750 and 1994 (Sabine et al., 2004b; Raven et al., 2005), with the lowest decrease (0.06) in the tropics and subtropics, and the highest decrease (0.12) at high latitudes, consistent with the lower buffer capacity of the high latitudes compared to the low latitudes. The mean pH of surface waters ranges between 7.9 and 8.3 in the open ocean, so the ocean remains alkaline (pH > 7) even after these decreases.
The consequences of changes in pH on marine organisms are poorly known (see Section 7.3.4 and Box 7.3). For comparison, pH was higher by 0.1 unit during glaciations, and there is no evidence of pH values more than 0.6 units below the pre-industrial pH during the past 300 million years (Caldeira and Wickett, 2003)12. A decrease in ocean pH of 0.1 units corresponds to a 30% increase in the concentration of H+ in seawater, assuming that alkalinity and temperature remain constant.
Hence we get the claim that “the ocean” has become 30% more acidic since the start of the industrial revolution.
Once the scare had been introduced, it grew legs and had to be nourished and in 2005, the Royal Society published a report entitled, “Ocean acidification due to increasing atmospheric carbon dioxide”.
The members of the committee producing that report included Dr. Ken Caldeira of the 30% claim, at that time at Lawrence Livermore laboratory. He was accompanied by scientists from the University of East Anglia, Southampton University and Plymouth Marine Laboratory, both the latter institutions are part of the UK Tyndall Centre for Climate Change Research, the main body in the UK promoting draconian emissions control on behalf of the UK government.
Gerald E. Marsh, Argonne National Laboratory (Ret) in a self-published paper, “Seawater pH and Anthropogenic Carbon Dioxide”, said of the RS report, “The Royal Society pH estimate for 2100 is thus consistent with a linear extrapolation of the eighteen years of data from Ocean Station Aloha. Such an extrapolation would appear to be unwarranted or questionable at best.”
He then mentions Calder and Wicket, probably not realising that the Royal Society report is essentially their paper re-hashed, with the main author sitting on the committee producing it. This time he concludes that:
…the eighteen years of Ocean Station Aloha or similar data appear to have been linearly extrapolated out to 2300. This is even more questionable than a linear extrapolation to 2100.
So the basis of all the hype is a calculation from an estimate, which gives a precise figure of 0.1pH decrease, they don’t even know the consequences of changes in pH, and the conclusions they reach are based on an extrapolation of eighteen years of data from one Pacific ocean station.