In questions of science, the authority of a thousand is not worth the humble reasoning of a single individual.
- Galileo Galilei
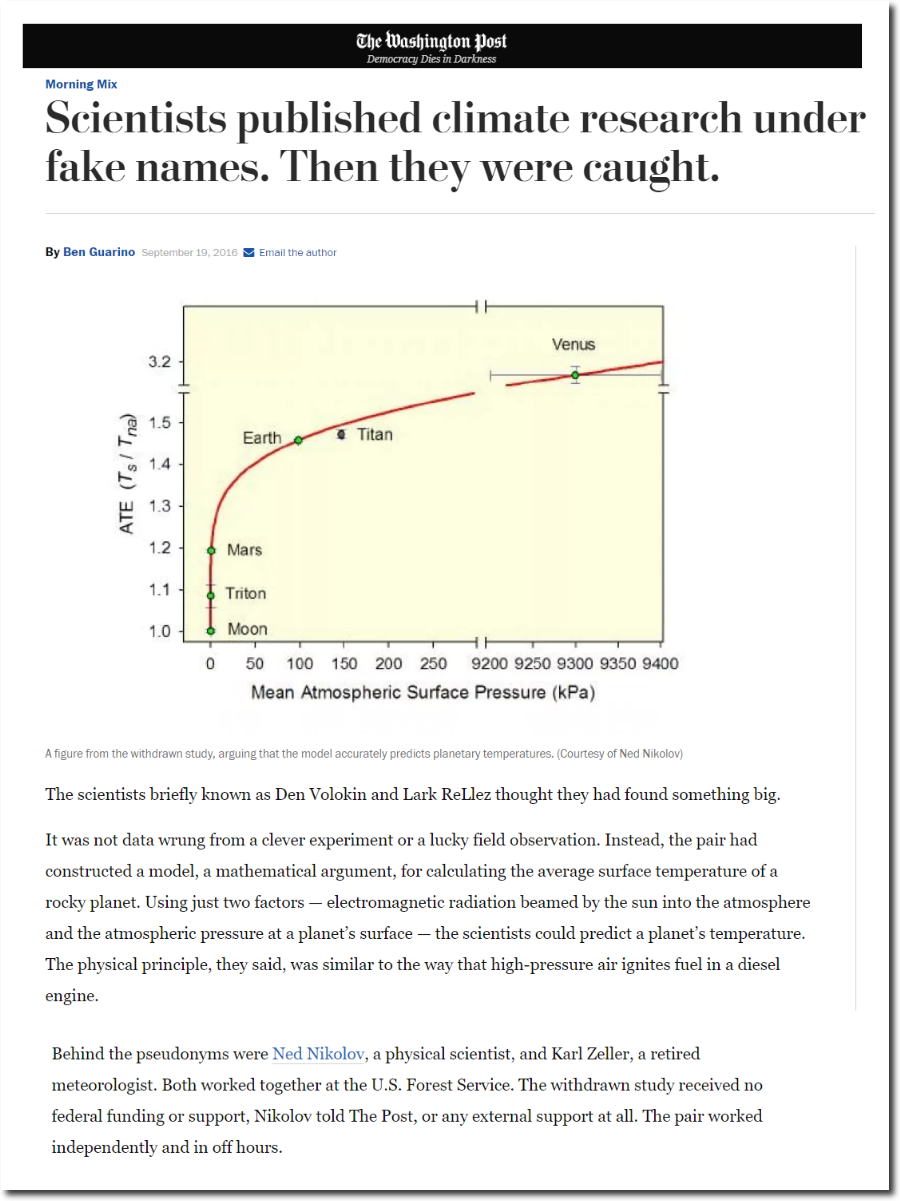
Ned Nikolov works for the US Forest Service in Fort Collins, Colorado. In 2016 he published the rather obvious observation that planetary temperature correlates with atmospheric pressure, not atmospheric composition.
Scientists published climate research under fake names. Then they were caught. – The Washington Post
While Obama was president, it was unacceptable for government employees to believe anything which strayed from the dogma of the global warming religion. Employees were implicitly threatened with termination for climate heresy. So Ned used a pseudonym, and the Washington Post ignored their research on that basis.
Sally Jewell: ‘I Hope There Are No Climate Change Deniers In The Department Of Interior’ | HuffPost
I have lost several jobs (immediately) after the company I was working for discovered I was a climate skeptic. No company wants to be called out and boycotted by green fascists. We live in dark times now – science and truth are for all intents and purposes illegal.
In a time of universal deceit – telling the truth is a revolutionary act.
– George Orwell