Before data tampering by NCDC, US temperatures show essentially zero correlation with atmospheric CO2. Climate sensitivity of zero.
Disrupting the Borg is expensive and time consuming!
Google Search
-
Recent Posts
- What’s At Stake?
- Too Hot To Live
- What’s At Stake?
- “The world began to end on 12th May 2024”
- “Climate change is a myth”
- Racist Gas
- RFK Jr. Discusses The Green New Deal And Climate
- “world is on edge of climate abyss, UN warns”
- Ivy Echo Chamber
- Climate Homicide
- Much Ado About Nothing
- Homophobic Gas
- World Bank Expectations
- Trained Not To Learn
- Protecting Endangered Species
- Record Climate Cynicism
- Record Cynicism
- The Latest In Climate Science Rhetoric
- Climate Desperation
- Artificial Stupidity
- Climate Obsession
- The Climate Empire Strikes Back
- Climate Scam Parrots
- “I’ve dealt with him before ”
- “I’ve dealt with him before.”
Recent Comments
- arn on “The world began to end on 12th May 2024”
- Billyjack on What’s At Stake?
- Disillusioned on What’s At Stake?
- Disillusioned on What’s At Stake?
- D. Boss on What’s At Stake?
- spren on Too Hot To Live
- Conrad Ziefle on “The world began to end on 12th May 2024”
- Conrad Ziefle on What’s At Stake?
- Bill on What’s At Stake?
- arn on “The world began to end on 12th May 2024”

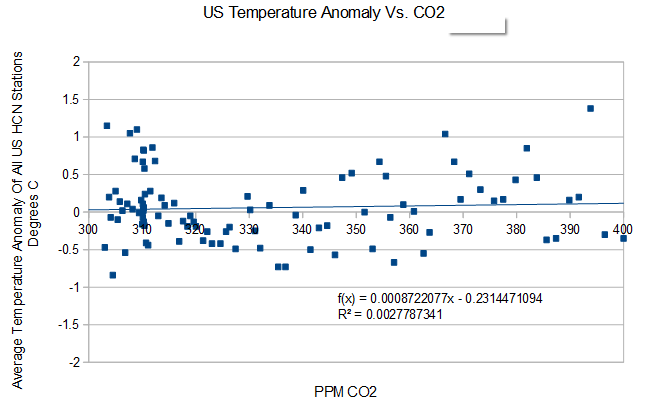

That is precisely the correlation they need to prove, and we are still waiting.
Agreed!. Reading all the IPCC related chatter shows the sensitivity is coming down. Its just far too slow for my liking and for policy makers to change their wasteful CO2-reduction based spending habits.
Without data tampering, there is no correlation. Thus the tampering.
Now, Steven, it is not nice to suggest that members of our most distinguished research institutions lied to the public!
They did, but their flaws will not solve our problems.
I am convinced, as 2014 draws to an end, that the salvation of humanity lies in the personal realization that powerful, but invisible, force fields from the Sun’s pulsar core are the same spiritual forces our ancestors worshipped at the dawning of each new day !
Happy New Year to All !
Can you imagine it – 50 years time when no one is left to protect the utter debacle and people are looking at this – what they will see is your statements of no correlation and it will look obvious to them that you were right.
And what they will wonder at, is how all the world governments and the vast majority of academics could have been so utterly wrong when someone like you or me could see the truth.
Surely, the fact so many people claiming to be “experts” got it so wrong despite the repeated attempts of people like me and you must have a very profound effect on these academics?
If there were a sensitivity to CO2 increasing, there would be a divergence between the middle troposphere temperature trend and the surface temperature trend. If there were a divergence between the two temperature trends, a determination of sensitivity could be back-calculated. But there isn’t a divergence and because there is no divergence between the two, one can only conclude that there is little or no temperature sensitivity to increasing CO2.
+1
The time element and its logarithmic effect makes this presentation very compelling.
Add in this graph of Tony’s and the evidence of fraud is complete:
https://stevengoddard.files.wordpress.com/2014/08/screenhunter_1618-aug-03-09-45.gif
Ah – the almost perfect correlation with CO2! Settled science!
Once again the time element tells the story; “cooling of the past.” It is evident in this image, you just have to take red pill and open your eyes.
As alarmists have told us, the US is not the world. Only places where there is no data, where expert “adjustments” can be made by climate scientists qualifies as the world.
I have lived in Colorado for over thirty years and nowhere else for over thirty years and the cold drop this day into afternoon was remarkable to say the least. Now back to the crawler and stuff a little more insulation into cracks.
Car thermometer says -15F in Fort Collins right now
Steve, you might be interested in this article at Ice Age Now.
Algeria[North Africa] – Snowfall leads to angry protests Seems they have a real mess on their hands. Be interesting to se if the US MSM mentions it.
This is AFRICA?
http://iceagenow.info/wp-content/uploads/2014/12/Algeria-30Dec2014.jpg
A station at Overland Trail & Poudre between Fort Collins and Laporte is showing -21.5°F.
It measured +8.1°F at 1:22pm, i.e. nearly 30°F drop.
Such a drop is “extreme weather” and clearly caused by global warming!
… and several other weather stations both north and south of Fort Collins also measure in the -20°-21°F range. Stay put and enjoy the balmy -15°F UHI in the city.
California is feeling winters bit too:
http://www.latimes.com/local/lanow/la-me-ln-126th-rose-parade-coldest-record-20141230-story.html
“This declining productivity of the ocean leaves in seawater is not bound carbon dioxide, the solubility decreases to the temperature rise of the Ocean (about one degree Celsius since 1910). Excess carbon dioxide is emitted into the atmosphere and its increasing concentration in sea water results in acidification of the ocean.
Ocean productivity is decreasing due to its decreasing fertilization, ie. Decreasing supplies from the depths to the surface layer of seawater silicates, phosphates, carbonates, iron, etc. Elements determining the continuation of photosynthesis, carbon dioxide binding with seawater. Lowering the productivity of the ocean is the result of a weak fertilization. Poor content life-giving elements in surface sea water is in turn the result of cosmic processes, but let’s talk about this turn.
The ocean is a biological machine and her life depends on the mixing of water in its depths. The process is as yet poorly recognized and is now marked by a lack of knowledge about the processes of ocean water exchange. Since life in the ocean is endless, it is clear that there is a circulation of water in its volume. It is caused by the constant and variable gravitational influence of the Moon and Sun on the density of ocean waters varied. Ocean tides occur on the surface and in the depths of the sea. Dense and cold water deep sea bottom sediments containing particles (including life-giving elements and dissolved minerals), escape to the surface cooling and fertilizing it, and oxygenated water surface and sink into the depths where oxygen support biological processes. Additionally upwelling (rich in silicates, phosphates, carbonates), the surface changes its acidity by neutralizing it. Water exchange between the depths and the surface is intensified by the constant changes in the position of pole-changing inclination of the Earth’s axis. This causes a change in the centrifugal force acting on the inertial mass of water and its movement horizontally and vertically in the oceans. Changing the position of the poles are due to changes in the geographical position of the Earth’s metallic core mapped location change of the magnetic poles. The kernel of gravity moves in a fluid under the influence of external kernel variable Sun’s magnetic field. When heavy metal core inside the Earth moves the liquid outer core is a change in the position of the center of gravity of the Earth and changing the position of the axis of rotation. This results in the geographical position changes polarity and as a result takes place under the influence of a variable centrifugal force, inertial motion of ocean water and mixing them in a volume’s ocean.”
ren,
This explanation makes more sense to me as a chemist.
http://www.co2web.info/ESEF3VO2.htm
(start with 6. Justifying the dogma – carbon cycle modelling vs. reality)
The ocean is buffered by the organics (life) and by the rocks like limestone and basalts.
Compare a map acidification and CO2.
http://earthobservatory.nasa.gov/GlobalMaps/view.php?d1=MY1DMM_CHLORA
“The highest chlorophyll concentrations, where tiny surface-dwelling ocean plants are thriving, are in cold polar waters or in places where ocean currents bring cold water to the surface, such as around the equator and along the shores of continents. It is not the cold water itself that stimulates the phytoplankton. Instead, the cool temperatures are often a sign that the water has welled up to the surface from deeper in the ocean, carrying nutrients that have built up over time. In polar waters, nutrients accumulate in surface waters during the dark winter months when plants can’t grow. When sunlight returns in the spring and summer, the plants flourish in high concentrations.
A band of cool, plant-rich waters circles the globe at the Equator, with the strongest signal in the Atlantic Ocean and the open waters of the Pacific Ocean. This zone of enhanced phytoplankton growth comes from the frequent upwelling of cooler, deeper water as a result of the dominant easterly trade winds blowing across the ocean surface. In many coastal areas, the rising slope of the sea floor pushes cold water from the lowest layers of the ocean to the surface. The rising, or upwelling water carries iron and other nutrients from the ocean floor. Cold coastal upwelling and subsequent phytoplankton growth are most evident along the west coasts of North and South America and southern Africa.”
El Niño therefore encourages a reduction ocean acidification.
ren,
I agree you are going to see minor fluctuations in pH dependent on the buffers, temperatures and mixing rate, however Catastrophic Ocean Acidification is not going to happen because there are too many buffering systems in the ocean.
The pH of the oceans are ~8.1 not acidic, not neutral but basic due to the buffers.
Guest Post by Professor Brice Bosnich at Jo Nova’s
The Chemistry of Ocean pH
Spurious correlations:
http://www.tylervigen.com/
“Researchers at the University of Miami and the National Oceanic and Atmospheric Administration (NOAA) studied the effects of acidification on fish larvae senses belonging to okoniokszta?tnych – Rachycentron canandum. These large tropical fish are very busy and popular with anglers.
With the method, computed microtomography (similar to that which is subjected to hospital patients), the researchers observed that in water at low pH fish develop a greater otolity (pebbles Labyrinthine) – included in the hearing – than animals from the waters of lower acidity. The weight of these structures made of calcium carbonate in the acidic water increased by up to 58 per cent., And a mathematical model for their functioning even indicated a 50 percent extension of the hearing.
“Increased hearing sensitivity allows the use of it to navigate, avoid predators or communication” – said one of the scientists Sean Bignami of the University of Miami.”
So the net greenhouse warming effect is near zero, just as I have been explaining in terms of the physics for several years. IR-active gases both warm and cool the surface with the cumulative effect near zero, at least as compared to the 33C effect the alarmists claim. The physical models offered up by the alarmists are wrong for the reasons I have been explaining. The experimental evidence confirms the wrongness of the physics in their models in a very decisive manner.
It is true that temperature does not correlate with CO2 concentration over short time scales (e.g the last 300 years) although you can always find a decade or two where coincidence supplies an apparent correlation.
Over periods of hundreds of thousands of years the correlation between average temperature and the concentration of CO2 is remarkable. However, temperature is driving [CO2] rather than the revesrse:
http://wattsupwiththat.com/2014/12/27/vostok-and-the-8000-year-time-lag/
Camel, is that correlation based on actual data or on CAGW adjusted data?
The original data from the ice cores and from historic wet chemistry methods showed much higher readings than the present ‘Generally Accepted’ values. Some of the data from the 1800 was as high as 500 ppm and above. Early Ice core values had a very wide concentration range of about 100-7400 ppm.
“Even more important was the finding that several physical and chemical processes (such as melting, the presence of liquid brines in the capillary-like interstitial voids, the presence of carbonates, over-pressure in the air bubbles, and solid deposition of super-cooled fog, combined with large differences in the solubility of different gases in cold water, and mobility of CO2 in ice) lead to differentiation of the original atmospheric ratios of N2, O2, Ar, and CO2, and to depletion or enrichment of CO2 in the ice (Coachman et al 1958; Hemmingsen 1959; Scholander et al 1961; Matsuo and Miyake 1966; Raynaud and Delmas 1977).”
In other words the bubbles in the ice do not capture ancient atmospheres and the readings are crap. This allows the same type of cherry picking that Callendar did to the early 18th and 19th century wet chemistry analysis of CO2.
Lucy Skywalker put together an excellent look at the history and as she said.
“Anyone challenging the official ice CO2 record has to be able to answer Ferdinand Engelbeen – which was where I started. I realized I needed to check back carefully with Jaworowski’s attacker Some Are Boojums (here and here etc). After studying this paper and Engelbeen, the inadequacy of his criticisms finally became transparent. A worthy challenger, however.”
http://www.greenworldtrust.org.uk/Science/Scientific/CO2-ice-HS.htm
She includes a copy of the following paper. she says “..may well be Jaworowski’s best paper. It is pure detailed science – whereas the science in his later papers becomes a little diluted by his righteous and passionate indignation….” I have to agree. As a chemist this is my favorite paper.
Do glaciers tell a true atmospheric CO2 story?
PDF of original paper: http://www.co2web.info/stoten92.pdf Z Jaworowski, T V Segalstad, & N Ono, 1992 227-284 Science of Total Environment
Also see: http://www.co2web.info/ESEF3VO2.htm
I should also provide links to Dr.Jeff Glassman’s excellent work
http://www.rocketscientistsjournal.com/2006/10/co2_acquittal.html
He also addresses my favorite point, the ‘well mixed’ conjecture is invalid.
(The [ ] are Glassman’s not mine.)
A later article addresses some of the attacks from the orthodoxy:
http://www.rocketscientistsjournal.com/2007/06/on_why_co2_is_known_not_to_hav.html#more
If the atmosphere is not ‘well mixed’ than none of the correlations between CO2 and temperature are valid. As Tony Heller has shown Clim-Astrologists make sure the correlation is there to support the CAGW dogma.
https://stevengoddard.files.wordpress.com/2014/10/screenhunter_3233-oct-01-22-59.gif
Reblogged this on Centinel2012 and commented:
Don’t let Hansen or Gore see this!