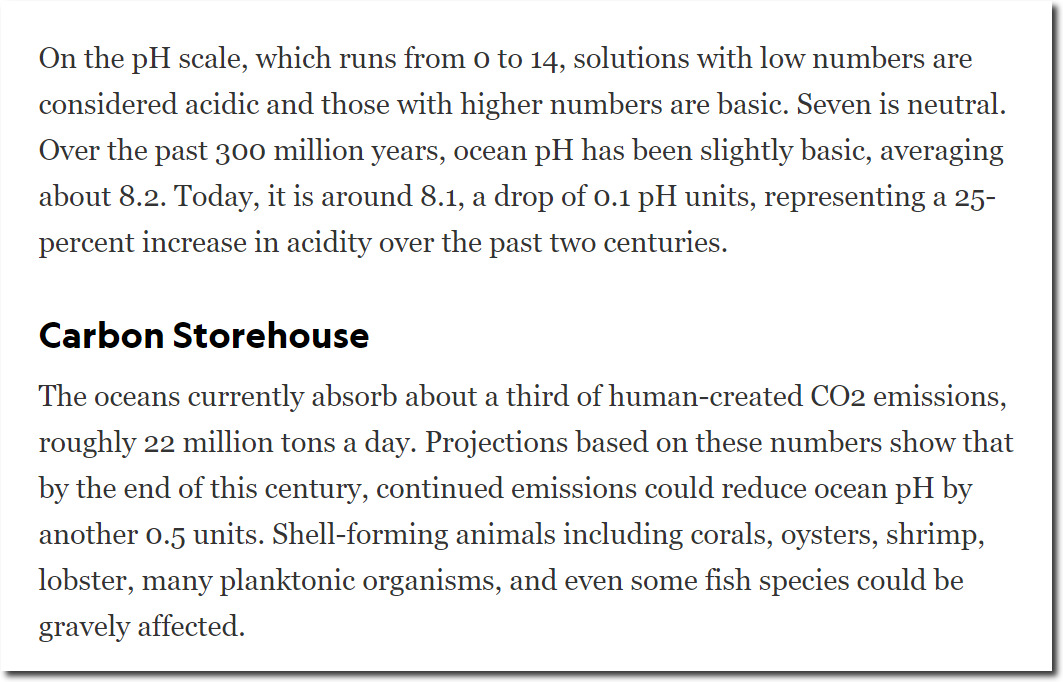
National Geographic say they have been monitoring ocean pH levels for the past 300 million years, and that the pH was a steady 8.2 for 299,999,800 years – until a little before you bought your SUV. Now they say the ocean is an “acid” 8.1 – and it is your fault. Corals are going to dissolve.
Ocean Acidification | National Geographic
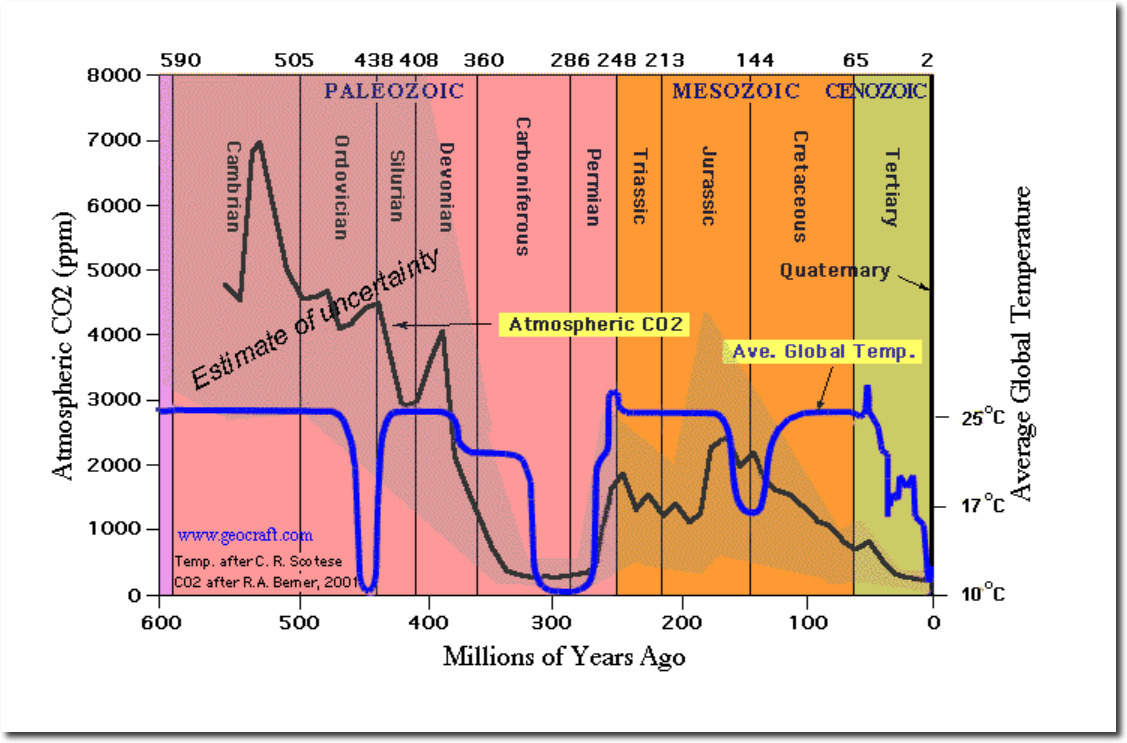
Atmospheric CO2 is close to the lowest level it has been in the last 300 million years. Corals evolved in the Cambrian Era – with CO2 levels fifteen times higher than now. Obviously they aren’t going to dissolve.
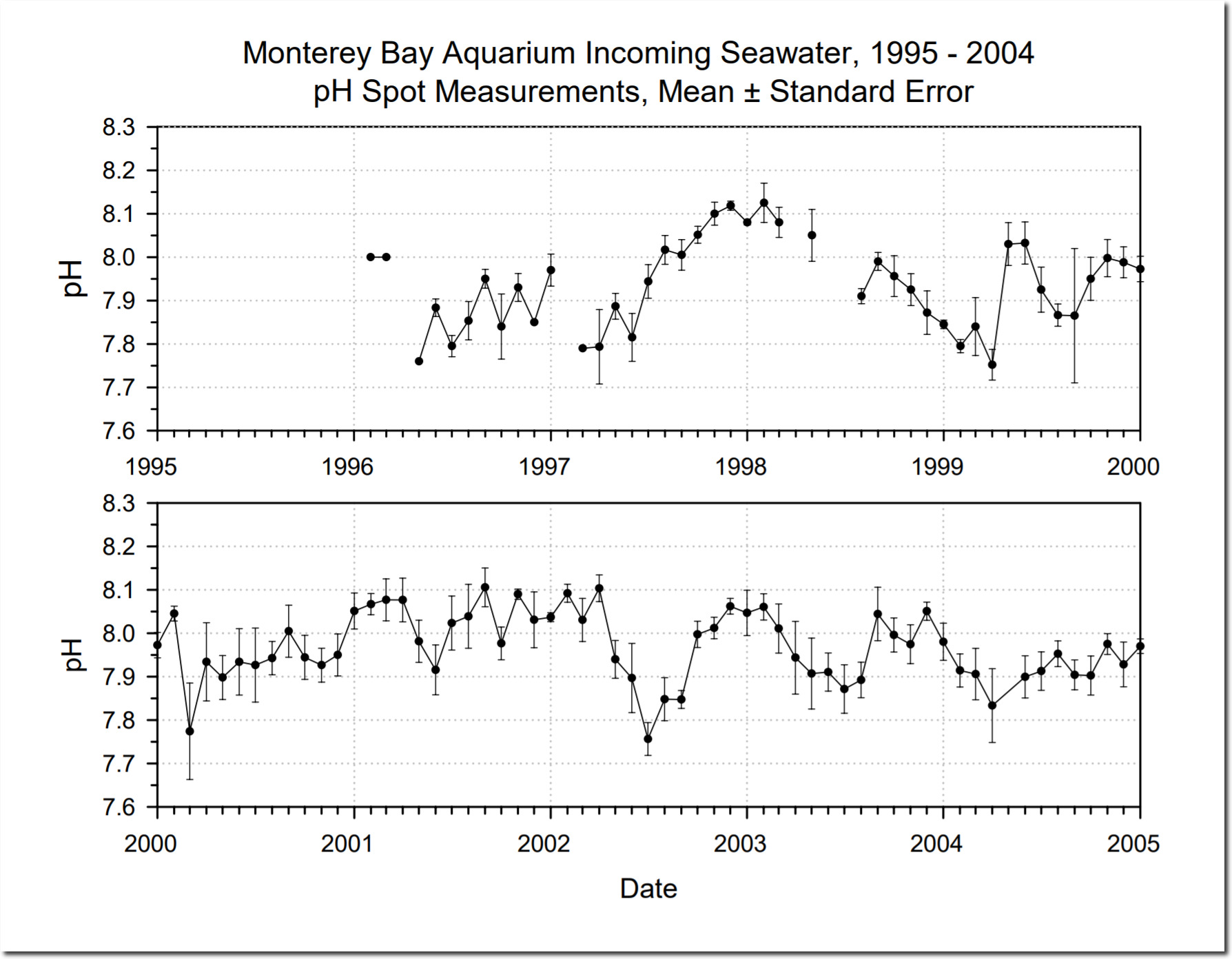
In the real world, seawater ranges from about an alkaline 7.7 to an alkaline 8.2.
National Geographic has no clue what they are talking about, and are simply making nonsensical gibberish up. But that is standard operating procedure for the left.