Disrupting the Borg is expensive and time consuming!
Google Search
-
Recent Posts
- Analyzing The Western Water Crisis
- Gaslighting 1924
- “Why Do You Resist?”
- Climate Attribution Model
- Fact Checking NASA
- Fact Checking Grok
- Fact Checking The New York Times
- New Visitech Features
- Ice-Free Arctic By 2014
- Debt-Free US Treasury Forecast
- Analyzing Big City Crime (Part 2)
- Analyzing Big City Crime
- UK Migration Caused By Global Warming
- Climate Attribution In Greece
- “Brown: ’50 days to save world'”
- The Catastrophic Influence of Bovine Methane Emissions on Extraterrestrial Climate Patterns
- Posting On X
- Seventeen Years Of Fun
- The Importance Of Good Tools
- Temperature Shifts At Blue Hill, MA
- CO2²
- Time Of Observation Bias
- Climate Scamming For Profit
- Climate Scamming For Profit
- Back To The Future
Recent Comments
- Bob G on Analyzing The Western Water Crisis
- Robertvd on Analyzing The Western Water Crisis
- Bob G on Analyzing The Western Water Crisis
- conrad ziefle on Analyzing The Western Water Crisis
- Bob G on Analyzing The Western Water Crisis
- Bob G on Analyzing The Western Water Crisis
- arn on Analyzing The Western Water Crisis
- conrad ziefle on Analyzing The Western Water Crisis
- Bob G on Analyzing The Western Water Crisis
- conrad ziefle on Analyzing The Western Water Crisis
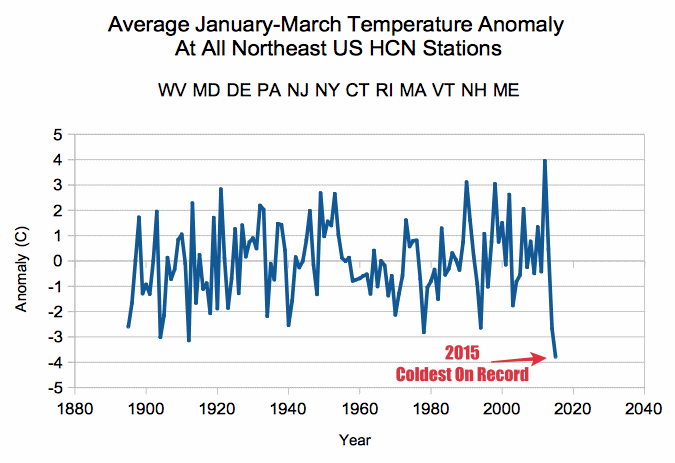
Cold January-March Is Weather. Warm December Is Climate.
This entry was posted in Uncategorized. Bookmark the permalink.



What is an Emmy Award for.. ?
Acting and TV front presentation.
Pretty sure its NOTHING to do with science. 😉
Master of the green screen?
He pretends to be looking at a map better than anyone!
Sad.. He wouldn’t have the job if not for his obedience to the ScYience…
ScYience!! ScYience !!!
Deep greens & blues are the colors I choose… green agendas and blue pills.
Rockabye sweet baby Eric.
Happy New Year one and all. Here is an off topic (sorta) gift.
Over at Tallbloke’s Talkshop is a great post. Humorous and it has a great graphic. it is about “Ocean Acidification” which the essay claims is the new scare story. It does not matter if one agrees with that, the essay is still worth reading. It is a hoot.
https://tallbloke.files.wordpress.com/2016/01/acid-bath.jpg?w=614
Tony Thomas: The Fishy ‘Science’ of Ocean Acidification https://tallbloke.wordpress.com/2016/01/01/tony-thomas-the-fishy-science-of-ocean-acidification/#more-24811
“You add acid or vinegar or something, and then watch the shells fizz and dissolve!”
Rolls eyes
The counter to that crap. (A test tube is NOT the ocean)
The oceans are highly buffered by calcium Ca++ (and magnesium Mg++)
Calcium is the main cation in river water, followed by Na and Mg, then K.
Dr. Tom Segalstad debunks the ocean “acidification” scare from a geochemical perspective.
Chemical Laws for Distribution of CO2 in Nature
More recently in the NIPCC Report
Gail,
Agreed, and the article is also a rebuttal to the bullshit, but the collectivist greens have been coming up with new scares for generations and the school system, press, and the “experts” have been able sell utter un-physical delusions time after time. I know for a fact that most kids in school (at least Florida kids) just accept that driving a car warms the planet. After all, even the luke-warm “skeptics” agree with the delusional “back-radiation” heifer dust.
Remember, the left is spinning myths and not doing science.
Just tossing out ammo that can be used in our fight for real science.
I am a crusader in high schools, my own little domain
Heifer dust …. 🙂
Thanks for that one Mark!!
Darryl, do you get pushback?
Our stomachs are full of hydrochloric acid, but our bones do not dissolve.
I wonder if these morons understand why?
“Our stomachs are full of hydrochloric acid, but our bones do not dissolve.”
Not well up on anatomy are you !!
I am very much “up on anatomy”.
Are you up on reading comprehension?
My reading comprehension ……97%
since when have bones been in direct contact with stomach contents ??
Maybe that’s why our Skeletal bones do not dissolve.
As you are “very much “up on anatomy”.” what do you think ??
Ingested bone will start to dissolve in the stomach but because of the shape & orientation of the human stomach it tends to pass though rapidly. Give a dog a bone a few days later dissect the dog-turds, inspect the bone particles …if you can find any. Some reading for you –
Lyman, R.L. 1994. Vertebrate Taphonomy. Cambridge University Press.
Jones, A.K.G. 1986. Fish bone survival in the digestive systems of pig,
dog and man, in D. Brinkhuizen & A Clason (eds.) Fish and Archaeology.
Oxford, British Archaeological Reports International Series 294: pp53-61
Gail, thank for Dr. Tom’s rebuttal
Ocean acidification has been my only concern with increased atmospheric CO2.
OCEAN CHEMISTRY is really very complicated, and I have found perhaps even more complicated than presented by him in some ways.
All of this goes to show a basic and marvelous tenant of the planet earth—-
—– There are many,many stressors continually causing the planet to go out of equilibrium, but then, there always seems to be methods of negative feedback causing a swing back to equilibrium
—–and to this I would like your comment—– I am increasingly of the belief that the feedback mechanisms may take many decades to occur and much the so called climate swings are due to what phase the sum of climatic oscillations are in
The game changer really exists outside of the earth, as in the sun and the various cycles.
One of the early studies on the effects of “ocean acidification” used hydrochloric acid to lower the pH, and then claimed that lower pH was dangerous to sea life. Science, huh? It seems that they did not understand that here in the real world, the oceans were not being changed by people dumping in HCl but were (theoretically) being endangered by CO2. You know… CO2. The same chemical that, once dissolved into the water, becomes one of the working materials from which the shells are actually constructed.
See http://www.seafriends.org.nz/issues/global/acid2.htm#scientific_fraud for details.
darrylb,
From all my reading my SWAG is
#1. The earth system is never in equilibrium. Instead it is ‘hunting’ for equilibrium with many, many cycles/oscillations.
#2. There are positive and negative feed backs.
#3 There are, at the present time (last few million years) two stable states with cold being the more stable . H/T Dr Robert Brown.
One of the biggest lies in ClimAstrology is TrainBreaths cartoon of a steady state earth and “Missing Heat” to get up to the present temperature.
#1. Are they looking ONLY at the present temperature, or do they take the average over the last few million years?
#2. Do they take into account the fact the young earth was a motlen ball and has since been cooling down slowly losing heat over time or do they start with absolute zero?
http://jonova.s3.amazonaws.com/graphs/lappi/65_Myr_Climate_Change_Rev.jpg
Starting hot with the heat capacity of the mantle, land and oceans factored in coupled with is going to get you to an entirely different temperature.
For that experiment with the sea shells, why not add tap water which is 1000% more acidic than sea water and see what happens?
Just make sure you add chunks of limestone first.
Since seawater is not an acid, it can be 1000 trillion times more acidic, and still not dissolve.
The pH scale has a centerline, and acids are on one side, and bases on the other.
An acid is defined in a certain way (actually, there are several definitions…Arrhenius’s, Lewis’s, Bronsted-Lowry’s…), but the climate liars do everything they can to render language and actual definitions meaningless.
The better to promote outright BS as science.
Menicholas, You completely misunderstood.
I was answering the comment:
“…For that experiment with the sea shells, why not add tap water which is 1000% more acidic than sea water…”
……………
Actually it completely depends on where the tap water is coming from. During a drought in the Boston area the Merrimac River derived tap water was chuck full of organic acids and had a low pH between 6 and 7 (It also smelled like a swamp — yuck!) Even with de-ionization it’s pH was acidic and caused havoc with the chemical batches the company was making that need a pH of about 8. Worse each batch tank of water was different. We had to titrate with a KOH solution to figure out how much KOH had to go into each different batch. It was a real PITA.
The chunks of limestone are just slow acting replacements for the KOH with a bit of sarcasm tossed in. Ocean basins are full of limestone and metals and the oceans full of buffers as a result.
In most cases, thanks to limestone, well water at least is alkaline (Hard). With river water, all bets are off thanks to the organic acids. (You will never drink city water again after you have had to change the filters on the city water line going into the DI system — GROSS!)
pH of Ground Water and City Water [Wisconsin] city water between pH 6.9 and pH of 8.4.
From the UN pH in Drinking-water – World Health Organization
Oceans, Lakes, Marshes and the like all have a bio-system which causes the water to be alkaline pH sometimes even close to 9.0
When lakes dried up as here in Minnesota in the 30’s and 50’s the lakes were too alkaline to farm, However, they were able to be used as pasture land.
I did not misunderstand, Gail.
I was just making a point about how acid base chemistry seems to be very poorly understood by many people. In particular, those who insist on calling any shift lower in pH “acidification”.
I am not usually pedantic about language, but in the case of so-called ocean acidification, I make an exception.
Menicholas, agreed. Acidification should be neutralization but it doesn’t cause panic like acidification does.
darrylb,
It depends. Rain water is acidic and creeks and rivers can have a wide range of pH from Acid to bas as a result of how much rain and what the river bed is composed of and as you said what the water biosphere is.
It looks like old man winter is here for the rest of the season Just as Joe Bastardi and the guys at Weatherbell have been predicting since last summer.
Just got back from a run to MA. Got my first little taste of winter driving this winter on Thursday. First ice buildup on the windshield wipers though I passed through the portion of I-90 in NY and PA before the lake effect snows hit. I hate being on the road on New Years Eve. Too many people trying to get someplace to party. There was a five mile back up on I-90 west bound at the junction with I-91 at 2:30 PM yesterday. Too many people heading for NYC I guess.
My posting has been limited. Still working to learn this new computer and get it set up.
I HAVE to get to Comcast to upgrade my cable modem from the 25 MPS I was using with my old computer to the 75 MPS that windows 10 apparently needs to function correctly. But work and New Year Holiday have conspired to prevent that from happening so far. Windows 10 is a whole new ball game for a guy that was using XP and it’s going to take awhile for me to figure it out.
Once I get the new modem and have that working and my home Network set up I will then start downloading all the accumulated data and links I had on my old machine onto this one. Then after that it’s getting the hardware for my TVs so I can use Chrome Cast to stream videos, movies, and music to them.
Then I will have to connect my Samsung S6 phone and my tablet to it. All this may be a snap for some but right now, this truck driver is kinda overwhelmed by it all and is playing catch up big time during upgrade.
Shudda got a Mac!
Dan Kurt
So true.
Hubby Junked the Windows and installed Linux on the machine we got last year. Will do the same if I ever get a new one.
Win10 doesn’t need that extra bandwidth to function and will make no functional difference other than downloads/uploads will be 3x faster. Win10 does not need extra bandwidth to function. And, I’d much rather have Win10 than a Mac. I’m an IT expert, btw, and have been working with Windows (and Apple/Macs) for >20 years.
Mac uses vastly superior Freetype fonts over Windows. If I want to do go graphics, I do it on the Mac
I rather enjoyed the warmer Fall and early Winter here in the Northeast, given, as Tony pointed out, our severe Winter / early Spring of 2014-2015.
I am just happy my kid crop (goats) was born in very nice weather and have been able to frolic in the grass for over a month. Now they are nice and big and ready to withstand nasty weather without me hauling the newborns into the house each night and then hauling them back out to mama for the day. (I really do not like heat lamps where goats could possibly get to them.)
I still have Taffy, a bottle baby from last year, making a dash for the open house door when ever she can. She gets in the truck too.
too funny….and way too cute!
You know, people used to celebrate weather like this. Now these a=holes have even turned that into some guilt trip.
No guilt or worries for me! I loved it while it lasted and it seemed to be that everyone else around here enjoyed it also. White Christmases are nice but I didn’t shed any tears about it being almost 60 deg. F on that day.
But our warmer than normal December is over and old man winter is here. I guess that means global warming is elsewhere right now.
It’s time for the under armor and poly pro underwear to come out and soon I’m sure I’ll will be shoveling and blowing snow from the deck and walks and the driveway and playing with the FJ on the snowy roads. And dealing with the winter in the big truck.
Now I gotta get dressed and load up some fire wood for the fireplace and then go get some ice melt and set it out in a 5 gallon pail by the back door for use because my wife took a tumble on our deck when it was frosted over one morning while I was gone.
We have our 13 month old granddaughter and she loves having a fire in the fireplace.
She touched the chainmail spark screen and it was warm enough that she figured out that it’s nice to be close to the fire but not too close. Just got to watch her when she is around it because she just isn’t steady on her feet yet.
Our last buck was the featured guest last night for dinner (YUM!) so I need a new buck.
It just so happens “…The USDA Regional Climate Hubs were established last year across the country, and the Southeast Regional Climate Hub (SERCH), in Raleigh, NC, is here to serve the agricultural climate change needs of the Carolinas (along with 9 other southeastern states)…”
I am REALLY looking forward to their help in finding a nice woolly cashmere buck to help winter proof my goat herd during the coming solar minimum….
https://media2.stickersmalin.com/produit/100/stickers-devil-smile-R1-143760-2.png
I just have to select which peer – reviewed papers I need to bring with me.
https://www.carolinafarmstewards.org/serch/
https://globalchange.ncsu.edu/serch/
Southeast Regional Climate Hub
920 Main Campus Dr, Suite 300
Raleigh, NC 27606
Quite a remarkable warm December, due to an unusual and persistent WEATHER pattern. No more indicative of climate change than the remarkably cold late winter WEATHER pattern this year that created a frozen and snow covered Potomac River in DC for the first time EVER in March.
The WEATHER is often doing something remarkable going back in history. That is why I have always found WEATHER history to be such an interesting and fascinating topic.
“…persistent WEATHER pattern.”
That’s the key – the persistence in this case was on the order of months, but then things cycle back the opposite way, just as El Nino cycles to La Nina. In fact, remember the endless drought that “climate change” was supposedly causing?? Guess what…
http://www.cpc.ncep.noaa.gov/products/analysis_monitoring/regional_monitoring/palmer.gif
Looks like California is almost back (and there is certainly no drought in the midwest!).
Frank, thanks for the graph. It is interesting to note the strong demarcation line along the Northern California Oregon border. Any clues as to why CA is near normal and OR very moist+?
My only clue, living here just above Yosemite, is that for most of Nov, Dec the jet stream took the storms further north. What moves jet streams?
What moves jet streams? — Solar UV (and cosmic ray) changes that change the ozone that change the amount of heat in the stratosphere among other things.
Penn State (snicker)
Ozone depletion trumps greenhouse gas increase in jet-stream shift (Note how they hook it to evil mankind )
NATURE: Jet-stream shifts linked to ozone
Quantifying the Summertime Response of the Austral Jet Stream and Hadley Cell to Stratospheric Ozone and Greenhouse Gases
And the Science Fiction put out by Climate Nexus “Changing the conversation”
http://climatenexus.org/learn/planetary-systems/jet-stream
The missing piece is the shift in the UV wavelengths of the sun.
Papers:
An influence of solar spectral variations on radiative forcing of climate
Climate sensitivity to the lower stratospheric ozone variations (Cosmic Rays also influence ozone)
Recent variability of the solar spectral irradiance and its impact on climate modelling (2013)
Mercy sakes, you should have heard the hysterics here in Western Washington this summer during a dryer than normal season. We were in a “drought.” The “drought” was caused by SUVs and their fossil fuels, etc. As someone who worked in a truly drought-stricken area, I was irritated by the hyperbole, but look at that big bad band of dark green in the upper left. It’s a miracle! The drought is broken! Don’t praise the Lord, praise Paris COP!
Yeah, right…
PDO and AMO drought correlation
http://sparkleberrysprings.com/v-web/b2/images/climate07/04mcabefig4lg.png
Explanation
http://appinsys.com/GlobalWarming/PDO_AMO.htm
PDO+ and AMO+ means buy corn futures!
Right now, just buy AMMO+
Looks like we are <a href="http://research.jisao.washington.edu/pdo/PDO.latest"PDO positive (2014 & 2015) and AMO heading negative which means rain for most of the USA.
PDO
http://research.jisao.washington.edu/pdo/img/pdo_latest.jpeg
YEAR JAN FEB MAR APR MAY JUN JUL AUG SEP OCT NOV DEC
2011** -0.92 -0.83 -0.69 -0.42 -0.37 -0.69 -1.86 -1.74 -1.79 -1.34 -2.33 -1.79
2012** -1.38 -0.85 -1.05 -0.27 -1.26 -0.87 -1.52 -1.93 -2.21 -0.79 -0.59 -0.48
2013** -0.13 -0.43 -0.63 -0.16 0.08 -0.78 -1.25 -1.04 -0.48 -0.87 -0.11 -0.41
2014** 0.30 0.38 0.97 1.13 1.80 0.82 0.70 0.67 1.08 1.49 1.72 2.51
2015** 2.45 2.30 2.00 1.44 1.20 1.54 1.84 1.56 1.94 1.47 0.86
AMO
http://www.cgd.ucar.edu/cas/catalog/climind/AMO_fig5.gif
Final data in on Arctic sea ice 2015….
Upward trend since 2007.
https://rclutz.files.wordpress.com/2016/01/masie-day-2015365r.jpg
..and the Antarctic was adding ice during that same time