The climate scamsters have known for about a decade that the global warming scam was going to collapse, so they developed a Plan B – ocean acidification. This would allow them to continue their destruction of the energy infrastructure, even after no one believed in global warming any more.
Check out this world class nonsense
Carbonic Acid
When carbon dioxide dissolves in this ocean, carbonic acid is formed. This leads to higher acidity, mainly near the surface, which has been proven to inhibit shell growth in marine animals and is suspected as a cause of reproductive disorders in some fish.
On the pH scale, which runs from 0 to 14, solutions with low numbers are considered acidic and those with higher numbers are basic. Seven is neutral. Over the past 300 million years, ocean pH has been slightly basic, averaging about 8.2. Today, it is around 8.1, a drop of 0.1 pH units, representing a 25-percent increase in acidity over the past two centuries.
Ocean Acidification — National Geographic
“Fifty percent of those pteropods are affected by acidification,” Bednarsek said. “It’s a lot—more than we expected.” And sooner. She tells me that acidification is happening sooner and on a larger scale than scientists predicted. “This is just an indication of how much we are changing the natural environment,” she said.
The study estimates “that the incidence of severe pteropod shell dissolution owing to anthropogenic [ocean acidification] has doubled in near shore habitats since pre-industrial conditions across this region and is on track to triple by 2050.” In other words, thanks to human carbon pollution, twice as many marine creature shells are dissolving as were before the industrial era. And three times as many will be dissolving by mid-century.
“By 2100, 50 percent of the oceans would no longer be viable for pteropods,” Dr. Richard Freely, the study’s co-author, told me, if we continue emitting carbon pollution apace. And that’s exactly what’s expected to happen.
The Pacific Ocean Has Become Acidic Enough to Dissolve Sea Snails’ Shells | Motherboard
How much crap can these people pack into a few short paragraphs?
1. A pH of 8.1 is alkali, not acidic
2. pH varies by far more than that from year to year, and gets much lower than 8.1 Monterey Bay regularly cycles between 7.8 and 8.1
sanctuarymonitoring.org/regional_docs/monitoring_projects/100240_167.pdf
The shellfish in Monterey Bay aren’t dissolving. According to the experts, they should all be dead.
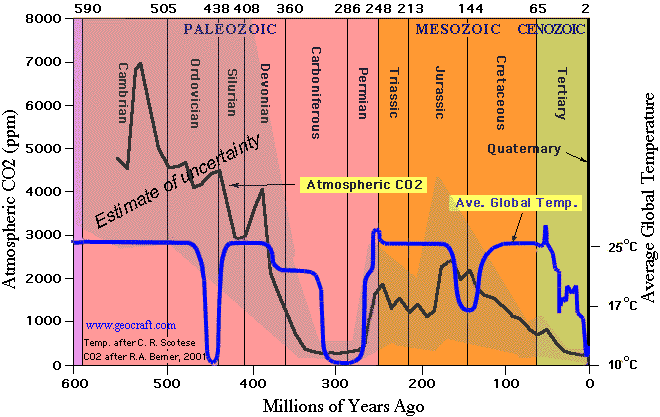
3. CO2 levels were much higher during most of the last 300 million years
They claim that higher levels of CO2 cause the pH to drop, and then they contradict themselves by claiming that pH was higher over the last 300 million years — when CO2 was also much higher.
In other words, they disproved their own theory. Higher levels of CO2 over the past 300 million years did not make the pH drop.
Unbelievable buffoonery from the people who make the Monty Python witch burners look like serious scientists. There may well be something affecting the shellfish – but it isn’t CO2. Does climate science “peer-review” actually serve any scientific purpose? They never seem to catch anything, no matter how blatantly incorrect it is.


Thanks in large measure to your hard-hitting responses to propaganda disguised as consensus science, Steven, we now know beyond doubt that the United States is ruled by deceit, exactly as George Orwell predicted in the book he started writing in 1946, “Nineteen Eighty-Four.”
Can we identify a realistic way to restore integrity to government science, sanity to society, and basic civil rights to citizens?
Political leaders may have “painted themselves into a corner” by training scientists to tell them what they wanted to hear.
The NAS and leading scientists are afraid they might be punished if they admit they lied to world leaders about nuclear and solar energy for the past seventy years.
So government deception will continue unless a rational way is identified to peacefully end this insanity.
Basically, the National Academy of Sciences violated its responsibility to the American public and to Congress by using its position as reviewer of budgets of federal research agencies for political purposes. If the current NAS President, Dr. Ralph Cicerone, can assure the new administration this abuse of science will not be repeated, the current system of budget review may be retained. If not, the new Congress will have to establish a new system of budget review.
Big Brother is going down now, thanks to Steven Goddard’s hard-hitting response to each of BIG BROTHER’S LATEST LIES:
https://stevengoddard.wordpress.com/2015/04/11/experts-say-that-ocean-acidification-killed-flying-insects/#comment-513947
I admire your optimism. I don’t have it.The borg is very powerful. The media are controlled. I can’t see how it will end.
But posts like yours give me at least a spark of hope that the insanity will some day be ending.
I agree, Disillusioned, the Borg has concentrated all human power in a futile attempt to hide the Force of Creation.
This is a re-enactment of the classic battle depicted in the scriptures of many religions. The battle between truth and falsehood; The battle between good and evil.
The conclusion to this paper shows the Force opposing the Borg: “Teacher’s Supplement to Solar Energy” https://dl.dropboxusercontent.com/u/10640850/Supplement.pdf
So relax and enjoy the show.
“Amazingly, the ocean response to more CO2 in the atmosphere is increased alkalinity and NOT increased acidity presumably because an increase in sunlight drives CO2 out of the oceans and into the air. The chart deals with the top 200 metres which is approximately the maximum depth to which sunlight can penetrate to any significant degree.
The oceans appear to have become more alkaline during the late 20th century warming spell and only since 1990 have they become more acidic as the warming stopped and according to Earthshine data global albedo has increased again.”
Comment by Stephen Wilde at WUWT, on March 31. to Michael Wallace’s post:
http://wattsupwiththat.com/2015/03/31/ocean-ph-accuracy-arguments-challenged-with-80-years-of-instrumental-data/
why would there be an increase in sunlight?
Fewer clouds.
The results of the latest NASA OCO-2 atmospheric survey satellite appear to have escaped the attention of Feely and Sabine, who appear to have based their results on the 2006 NASA
computer gameclimate model of atmospheric CO2 distribution. Note how thegamemodel shows the vast majority of the atmospheric CO2 to be resident in and emanating from the Northern hemisphere.https://i.ytimg.com/vi/x1SgmFa0r04/hqdefault.jpg
Unfortunately for Feely and Sabine (and very many other AGW researchers), the first results from the OCO-2 project show a vastly different picture.
http://www.nasa.gov/jpl/oco2/nasas-spaceborne-carbon-counter-maps-new-details/#.VMZutnDkfxp
http://www.nasa.gov/sites/default/files/thumbnails/image/mainco2mappia18934.jpg
Clearly, the majority of atmospheric CO2 concentration is in the Southern hemisphere, much appears to be produced by the rain forests, and a considerable amount appears to originate from the Pacific ocean. The Northern hemisphere on the other hand is responsible for relatively little of the atmospheric CO2, the British Isles in particular seem to produce below average concentrations.
Basically, liquids absorb gases according to a linear relationship in accordance with Dalton’s law whereas with increasing temperature liquids emit dissolved gases in a non-linear fashion dictated by Henry’s law. For example, if water is placed in a vessel containing an atmosphere of 100%, as it warms it will release the CO2 and at 100°C will not contain any dissolved CO2 at all.
So effectively, warming oceans and ocean “acidification” are mutually incompatible, note that the NASA OCO-2 map shows that the cold Southern ocean is the only one that appears to be actually absorbing CO2, all the other (supposedly warming) oceans are either neutral or emitting it.
Anyone who has ever opened a warm bottle of soft drink can tell you that warmer water will only hold dissolved CO2 under pressure.
Peer review.
A skill learned in early childhood is to seek permission, support or approval from someone likely to grant it.
Peer review.
Non-NASA Non-Scientist Steve Goddamn Presents Unequivocal Proof That He Doesn’t Know What He Is Talking About. A PH of 8.1 may alkaline, but it is 25% more acidic that 8.2%. You are obvious incapable of scientific subtleties. You know nothing about science, but clearly live in profound fear of the great Red Scare that obviously traumatized you as a youth.
You have been awarded prat of the year 2014. Whatever % change there MAY have been on AVERAGE the oceans and seas remain ALKALINE. AND for the sake of assole idiopts such as yourself an alkaline ocean is many times more dangerous than an acicidic ocean.
Experiment for the idiot. 7 is neutral. OK with that idiot ? Good. Now move 3.7 points into acid and drink it. Still alive. Good now make a liquid of 10.7pH and drink it. No, no you better not, I didn’t mean it you half wit.
3.3pH lemon juice. 10.7 bone dissolving slaked lime.
Children like you should not be allowed out alone.
“A PH of 8.1 may alkaline, but it is 25% more acidic that 8.2%. ”
8.1 is 25% more acidic than 8.2?
Where did you learn acid/base chemistry?
pH is a measure of the hydrogen ion concentration of a solution, and is calculated on a logarithmic scale. It is equal to minus the log of the hydrogen ion concentration.
Since water exists in equilibrium with H (present as hydronium ions) and OH, and the product of these two is always equal to 10^-14 ( meaning that the self ionization constant of water is 14^-14), a pH of 7 means that the hydrogen ion concentration is 10^-7.
pH of 8.2 corresponds to a hydrogen ion concentration of about 6.3×10^-9
pH of 8.1 corresponds to a hydrogen ion concentration of about 7.9×10^-9
In any case, since neither of these pH values is in the range considered to be acid, to say one is more acidic than the other is nonsense.
One is less basic than the other. More neutral would be perhaps a more descriptively accurate way to say it, since these values are barely basic at all, considering the range of values an aqueous solution can have.
Can you explain where you get 25% from these numbers, or are you just repeating something that someone else said, and doing so out of the context of their remark?
Correction:( meaning that the self ionization constant of water is 10^-14)
Yep the progressive doom machine from so called “scientists” to the likes of Istasz continue to show they cannot even master the terminology associated with the pH scale. High School level stuff. Above 7 it is more or less basic/alkaline. At 7 Neutral. Below 7 it is more or less acidic.
Hell, rain water is normally slightly acidic! So I guess all those mollusks that hide out in the sand, like the clams humans and hominids have dug as hunter gatherers during low tide over the millennia should have melted away during monsoons? And fresh water clams shouldn’t exist at all.
For the math challenged here (applies to most everyone on this site) here’s the rudimentary math (that embarrasses me to have to work out for you).
Using your own numbers,
(7.9 – 6.3)/6.3 ~ 25%
So a change of pH from 8.2 to 8.1 represents a 25% increase in acidity. Doesn’t get much easier than that, boys.
My observations about the pervasive red baiting, not-so-subtle racism and pathetic Tea Party mentality (or lack thereof) on this site is a sad statement on the Fox news crowd.
Heh. You speak like one of the Kos Kids. Or are you an intellectual heavyweight on the Democratic Underground? The clichés are oozing out of you like you graduated from the Party young cadres school.
And you don’t understand the chemistry behind your 3rd grade calculations.
Come back soon and entertain us again.
Now calculate how much further percentage change is required before it even neutralises.. you know pH = 7
Then prove that there has been this change from 8.2 to 8.1 anyway. Its a made up change, fabricated in a model.
And then explain how the calculated the pH of the ocean 80 years ago, and what the margin of error is.
Sorry, it seems that istasz = ignorance personified… a mathematical and scientific non-entity.
What percentage increase in acidity would be necessary to lower the pH from 8.3 (start) to pure, neutral, distilled water? pH 7.0
2000%
And again, for something to become ‘more acidic’, it must first be ‘acidic’.
Go ban dihydrogen monoxide, moron. 😆
https://www.youtube.com/watch?v=yi3erdgVVTw
https://www.youtube.com/watch?v=8L6KGuTr9TI
He should change his handle to Acidistasz before another sufferer with carbon dioxide-phobic disorder grabs it.
Because I know the imbecilic Istasz won’t answer,…
… under his calculation method, a further ? 1200% change in H+ ions is needed to reach neutral pH.
And ffs, what was that ‘~’ he used meant to be?
A further illustration of his mathematical ignorance?
Or a further illustration in his ignorance in the use of computers ?
Both.
I should have read this thread before I said much the same thing on a later thread.
Menicholas explanation is correct.
Saying something is more acidic when it is really less basic (alkaline) is a misrepresentation of terms meant to confuse the truth and anyone with a scientific background finds that distorting a truth for the purpose of trying to confuse readers
is quite wrong. In one sense it could be considered more acidic, but it is highly misleading for a wrong purpose in mind, which is to make people think the oceans are now acidic. They are not.
Rainwater has a pH between 5 to 6 and you will note that they are referring to ocean pH close to the shores.. Estuaries, which are heavily influenced by rainwater often have a pH in the acid range. Water run off, due to land usage, unfortunately has been faster and limestone and the like (alkaline in nature) over which the water moves has been diminished. That fact is an environmental problem.
I have been trying to note how ocean pH has been measured. It is difficult to do for many reasons, and I am finding that it may be suspect.
But like in one time in our history we blamed everything on witches, we now blame every thing on CO2. In the end, doing so, that is barking up the wrong tree, will often hurt the environment. We must be as accurate as we can in explaining any facts and not try to distort them.
istasz says:
“For the math challenged here (applies to most everyone on this site) here’s the rudimentary math (that embarrasses me to have to work out for you).
Using your own numbers,
(7.9 – 6.3)/6.3 ~ 25%
So a change of pH from 8.2 to 8.1 represents a 25% increase in acidity.”
So you are embarrassed at someone who is challenged by rudimentary math, and yet you botch the formula for calculating a percent change?
Others below have pointed out that this calculation is sophistry at best, but even at sophistry you fail.
Which makes you either a total moron and a jackass, or a conniving liar who thinks that proving a point by using a made up formula will make you look smart.
In case you are just a moron and a jackass (It is impossible to say for sure, given the available facts. Conniving liar is a distinct possibility), here is the correct formula for calculating a percent change:
“In mathematics, the concept of percent change is used to describe the relationship between an old value or quantity and a new value or quantity. Specifically, the percent change expresses the difference of the old and new values as a percentage of the old value. In general cases where V1 represents an old or initial value and V2 represents the new or current value, percent change can be found with the equation ((V2-V1)/V1) × 100. Note that this quantity is expressed as a percentage.”
There are other ways to phrase this general form, but they all amount to the same thing, and they all have one important thing in common, which is that THE ORIGINAL VALUE IS PLACED IN THE DENOMINATOR.
I will provide a link to a site which will allow you to study this and other related concepts, and also has some exercises for you to practice on while you work to un-embarrass your own foolish self.
Back to school, doltish buffoon.
http://www.wikihow.com/Calculate-Percent-Change
By your logic, green would be 20% more blue than red. This is of course confusing gibberish, very much the same as using words like “acidification” to describe a situation which should correctly and honestly be described as “neutralization”.
The word “acidification” means literally, to make acid. But acid is not being made anywhere in this circumstance. None. Not a drop.
“By your logic, green would be 20% more blue than red”.
I hope I can stop laughing soon, I have to drive to the store!
I think some milk came out of my nose.
And I was not drinking any milk!
And 5 would be more negative than 10!
That actually is how color theory works though.
Moronic Idiot, and lame-brain, non-entity, Istax, sprouts CRAP !!
Has ZERO idea of log pH.
The tiny change you mention is a MODELLED change built on scientific ignorance.
Even with that tiny change , which is well within ANY measurement capability, why don’t you work out what further percentage change is required to even reach pH 7.
You are a GULLIBLE , non-scientific clutz !!!
“You know nothing about science”
Coming from a scientific MORON like you, that is quite ironic.
FFS, go back and pass junior high science before you bother posting again, you are embarrassing yourself.
https://www.youtube.com/watch?v=C_Kh7nLplWo
istasz says: April 11, 2015 at 7:03 am
Next time you want to enter another dissertation on ‘scientific subtlety’ into the record, find a good stand-alone place for it. You need to learn which reply button to hit. Only a scatterbrain could think your scholarly contribution had something to do with the preceding comment.
NOAA would seem to disagree with you concerning the use of the term ‘acidificatiion’.
pH values above 7 are commonly referred to as “basic” (or “alkaline”).
These common terms engender confusion, because a pH value does not directly reflect a quantitative measure of the concentration of bases in the solution, nor do high pH values constitute a measure of alkalinity. What is expressed by pH values >7 is still the acidity of a solution, it’s just that the acidity (H+ concentration) is very, very low (less than 10-7 (or 0.0000001) moles per liter, to be specific). To determine the alkalinity of a solution (which is related to the concentration of bases), a separate, detailed laboratory analysis must be run on the solution, so it is incorrect to characterize the change in hydrogen ion concentration as a decrease in alkalinity.
Calling this phenomenon “ocean acidification” when surface seawater will remain “basic” under future emissions scenarios is alarmist
http://pmel.noaa.gov/co2/story/A+primer+on+pH
Now, let’s see, who to believe, the scientists at NOAA, or a sad, scientifically illiterate, highly abusive troll on a blog…
It’s a puzzle.
How about instead of the highly biased political appointees at NASA, we use a definition which has not been purposely contrived to lend credence where no credence is due.
Say instead we use the definition of the term from any dictionary one may find on any library shelf in the world, or perhaps the definition from a chemistry textbook?
How about believing in what is true, rather than in what supports a clearly unsupportable attempt to arouse fear in the ignorant and the gullible?
Let’s see, how about that?
And it is no puzzle. Your transparently coy posturing notwithstanding.
Webster Dictionary
1.Acidification(noun)
the act or process of acidifying, or changing into an acid
Origin: [Cf. F. acidification.]
Princeton’s WordNet
1.acidification(noun)
the process of becoming acid or being converted into an acid
Wiktionary
1.acidification(Noun)
The act or process of making something sour (acidifying), or changing into an acid.
Oxford
Definition of acidify in English:
verb (acidifies, acidifying, acidified)
Make or become acid:
[with object]: ‘pollutants can acidify surface water’
Derivatives
acidification – noun
Cambridge Online
(Did not contain entry for acidification, but did have the verb form, acidify)
acidify
verb [I or T] uk /??s?d.?.fa?/ specialized us
to become an acid or to make something become an acid
As for this assertion:
“These common terms engender confusion, because a pH value does not directly reflect a quantitative measure of the concentration of bases in the solution, nor do high pH values constitute a measure of alkalinity.”
This contradicts the definitions given in all or nearly all texts on the subject, as well as what is commonly understood to be conveyed by the use of the term.
Moreover it attempts to redefine a term without acknowledging the commonly accepted usage, arguing as if every previous discussion on the subject of acid base chemistry which has ever occurred was in error because they did not check with NOAA first.
Such semantic gymnastics are sophistry at it’s most callow.
One can verify with sources old and new, lay definitions and those given in professional dictionaries:
http://www.britannica.com/EBchecked/topic/454823/pH
“PH
Chemistry
Written by: The Editors of Encyclopædia Britannica ? 1
Alternate titles: hydrogen ion concentration; potential of hydrogen
pH, quantitative measure of the acidity or basicity of aqueous or other liquid solutions. The term, widely used in chemistry, biology, and agronomy, translates the values of the concentration of the hydrogen ion—which ordinarily ranges between about 1 and 10-14 gram-equivalents per litre—into numbers between 0 and 14. In pure water, which is neutral (neither acidic nor alkaline), the concentration of the hydrogen ion is 10-7 gram-equivalents per litre, which corresponds to a pH of 7. A solution with a pH less than 7 is considered acidic; a solution with a pH greater than 7 is considered basic, or alkaline.”
http://www.merriam-webster.com/medlineplus/pH
“Main Entry: pH
Pronunciation: \(?)p?-??ch\
Function: noun
: a measure of acidity and alkalinity of a solution that is a number on a scale on which a value of 7 represents neutrality and lower numbers indicate increasing acidity and higher numbers increasing alkalinity and on which each unit of change represents a tenfold change in acidity or alkalinity and that is the negative logarithm of the effective hydrogen-ion concentration or hydrogen-ion activity in gram equivalents per liter of the solution ; also : the condition represented by such a number—compare”
http://www.chem1.com/acad/webtext/acid1/abcon-2.html#PH
“In a neutral solution at 25°C, pH = pOH = 7.0. As pH increases, pOH diminishes; a higher pH corresponds to an alkaline solution, a lower pH to an acidic solution. In a solution with [H+] = 1 M , the pH would be 0; in a 0.00010 M solution of H+, it would be 4.0. Similarly, a 0.00010 M solution of NaOH would have a pOH of 4.0, and thus a pH of 10.0. It is very important that you thoroughly understand the pH scale, and be able to convert between [H+] or [OH–] and pH in both directions.”
And then there is this:
“To determine the alkalinity of a solution (which is related to the concentration of bases), a separate, detailed laboratory analysis must be run on the solution, so it is incorrect to characterize the change in hydrogen ion concentration as a decrease in alkalinity.”
Whomever wrote this seems unaware of the reciprocal nature of the pH and the pOH scales, or the self ionization of water which relates them in a simple equilibrium at 10^-14.
Incredible.
Correction: “How about instead of the highly biased political appointees at NASA”
Should of course be: “How about instead of the highly biased political appointees at NOAA.”
Sorry.
To become more acidic, they’d have to be acidic in the first place.
“So a change of pH from 8.2 to 8.1 represents a 25% increase in acidity. Doesn’t get much easier than that, boys.”
Monumental stupidity from istasz,since it is NEVER acid at all. 8.2 and 8.1 numbers are strongly Alkaline factors.
What we ought to be saying, is that ( if the modelled change is real, lol! ) then..
The oceans have become LESS CAUSTIC !!
My grandmother regularly scolded me about various peer-approved activities during that age of exploration most of us went through. When I did—again—something indefensibly stupid, I habitually retreated to the argument that other kids were doing it, too. It took me some time to learn that just like some fatal weak moves on a chessboard it always lead to my defeat as she terminated my pathetic maneuvering by her queenly coup de grâce:
“If the other kids jumped off the bridge, would you have jumped, too?”
It seems we should stop giving money to certain scientists—at least until they grow up.
———-
P.S. I like the spelling bee rhythm of your putdown. It gives it that air of grownup authority. I will test it on the next smug alarmists that bring up peer review. Since they are resistant to logic anyway it’s only fair to use rhetorical devices against them and yours is very neat.
Peer review is completely dependent upon whose work is being reviewed. The peer of a circus clown, is a circus clown.
If the party whose work is being reviewed is a believer in CAGW, then so are his peers.
Yes but some clown-on-clown peer reviews are frowned upon:
https://www.youtube.com/watch?v=bIM4nxvepGI
Please do not denigrate rodeo clowns by comparing them to killer clowns…
http://www.freakingnews.com/pictures/106000/Barack-Obama-Clown-with-a-Gun-in-Moscow-106246.jpg
https://facistfunnies.files.wordpress.com/2009/09/evil-clowns-poster.jpg?w=595
Since my remarks got separated by several vertical feet here is what I was commenting on:
A good friend of mine once put it this way, when alarmists were pulling their hair out over a possible 30% change in ‘acidity’…
30% rise in acidity!? A solution of pH[1] has 100,000,000,000,000 times more hydrogen ions (acidity) than a solution of pH[14]. If I had a solution at pH of 8.5 the hydrogen ion content would be 3.2 x 10exp(-9) M. A 30% increase in hydrogen ion content is 4.2 x10exp(-9)M. Converting this to pH becomes… wait for this… 8.4!!!!! Yes you guessed it, nothing to write home about. It doesn’t sound half as threatening as 30% does it?! Kinda’ makes a mockery of percentage with respect to pH…
chemistry.about.com/od/chemistryquickreview/a/phreview.htm
Now I know I’m just an evil oil shill lackey, scientifically moronic, ununderstanding, conspiracy driven republican pontificating the tea party line… but… what percentage increase in acidity would be necessary to lower the pH from 8.3 (start) to pure, neutral, distilled water? pH 7.0
Want a hint? 2000%
Beware of the giant midgets! 😆
Ah yes, our innumerate friends the warmistas. Largely limited to word games, the pH scale eludes them. Ocean Acidification now that has a real ring to it. Never mind it means in practical terms just about nothing.
When I hear these parrots squawking about Ocean Acidification, I am always reminded about this demonstration…
https://www.youtube.com/watch?v=yi3erdgVVTw
Dopes, one and all.
From a WUWT post….
…Many people think that the ocean has only one pH everywhere. Other people think that the oceanic pH is different in different places, but is constant over time. Neither view is correct.
First, here is a view of a transect of the north Pacific ocean from Alaska to Hawaii, with Hawaii on the top left, Alaska on the top right, and depths shown vertically. ocean ph along transect
https://wattsupwiththat.files.wordpress.com/2010/06/ocean-ph-along-transect.jpg?w=720
Figure 1. Variation in pH by latitude and depth. The graphic is taken from a previous post of mine regarding oceanic pH.
Note that in Hawaii, the surface pH is above 8.05, and in Alaska the surface pH is below 7.7 … and despite that, the marine environment in Alaska is much, much richer in life than the Hawaiian marine environment. This underscores a simple fact—alkalinity is hard on living creatures, much harder than acidity. For example, if you want to dissolve the victim of your latest murder spree, you’d use lye (a strong alkali) and not sulfuric acid (a strong acid). [Well, maybe not you, but your neighbor about whom everyone always said “He always seemed like such a nice man …]
—————————-
istasz, you sound arrogant, ignorant and angry. Do you neighbors think you nice? You completely miss Tony’s main messages, and bring up an irrelevant straw man which Gator showed you was simply irrelevant. There are many studies showing the projected harms in this alarmist article and paper are simply wrong. Do you wish to discuss more?
There is no acidity in your diagram. None at all.
The choice for marine organisms is more alkaline or less alkaline, and of course neutrality is relatively benign compared to a strong alkaline.
There is no “ocean acidification”, it’s an outright lie. Oceans are everywhere and always alkaline to a greater or lesser extent; the CO2 might be (very slightly) neutralising this, so the honest term would be “ocean alkaline neutralization”. As is the case with temperature, the natural variation in pH is several orders of magnitude larger than any measurable trend… to the point where the trend may not even really exist.
Tel says, “There is no acidity in your diagram. None at all.”
=================
Yes. Did anything about the post make you think it was alarmist?
Link?
Excellent post, David. People often miss the point that CO2 will dissolve in greater amounts of water that is cold and under pressure. So a local upwelling of a deep ocean current can bring up water that has more CO2 and a lower pH (still not acidic).
So measuring the pH of the oceans has many of the same issues that measuring the temperature of the earth does, subject to the same manipulations to support a cause that is not science.
The 25 or 30% exaggeration assumes that H+ is the only species of importance in this case, when in fact HCO3- is the determining species for acidity of this solution. Going from 8.2 to 8.1 pH changes the acidity (the ability to titrate a base) by a few percent.
The pH of freshwater lakes and rivers is almost always below 7, and there are fresh water mollusks and crabs, and fresh water corals, that have no problem with their carbonate shells.
Acidification is all just a crock of rocks.
It does not sound very scary to say “The ocean is getting more neutral!”
Or even “The ocean is getting less basic!”.
Not only is the claim BS, but the way they spin it is BS.
Bullshit squared!
““It’s a lot—more than we expected.”
Oh no you didn’t.
Oh no you did not!
There are places in the ocean with extremes of temperature and pH, and many of these exist right alongside rich biomes of aquatic life. Black smokers are generally surrounded by dense communities of shellfish and other forms of aquatic invertebrates. In other places there are hydrothermal vents associated with volcanoes, and coral and shellfish are living and thriving nearby. The idea that small changes in pH would somehow be deadly to vast swathes of aquatic life is hogwash. If this was the case, floods and volcanic eruptions and other events which caused drastic and rapid fluctuations in pH and temperature would have long since wiped out these supposedly delicate species.
I would have thought that the evolution of calcium carbonate shelled oceanic organisms and huge areas of coral reef limestone built up through the Paleozoic era would suggest that there might be something wrong with the theory that high levels of carbon dioxide cause acidic oceans that are fatal to such creatures. Oh for a bit of basic geology teaching in schools! One field trip to the Much Wenlock Silurian limestones would be enough in the UK; I’m sure you have equivalent formations in the USA. There was a huge flowering of shelled life after the Ordovician extinction, when CO2 was at least 10 times what it is now. And corals, crinoids, brachiopods, and other groups which were alive then still exist today, which makes me wonder how anyone can believe that a miniscule extra bit of CO2 in the atmosphere, with or without a bit of extra warmth, can lead to a disaster for all life on earth. I truly despair sometimes.
Its called buffering.. something that alarmist non-scientists seem to know ZERO about.
There is no way to any acidification can happen in the oceans without first dissolving the limestone and basalt in or around the oceans.
The natural state of the oceans is around 8.1 +/- a bit, and all the slightly acid rain and the slightly more acidic rivers that have flowed into those oceans over millions and millions of years have not affected the ocean pH one little bit.
Certainly, no tiny change in atmospheric CO2 is going to have any effect what-so-ever.
Considering that we dump gazillions of galitres of ph 8-13 of soaps, bleaches, cleaning chemicals and so on into the oceans through sewerage (even after treatment the ph is over 7) then some CO2 in the ocean is a good way to prevent our soap dumping from deacidifying the ocean by dangerous amounts
Seen that graph a number of times. Never noticed before, there appears to be a regular drop in temperature every 150 million years.
For 100’s of millions of years, all the slightly acidic rain that has fallen on the seas…
and all the somewhat more acidic rivers have fed into the sea…
yet the sea remains staunchly ALKALINE, around 8.1 pH
ANYONE that thinks a tiny change in atmospheric CO2 level is going to have any effect, needs to be put into an moron asylum !!
They can join Trenberth, Mann, Romm, Obama et al etc !! and won’t they all enjoy that !!
Former ruler of Australia, Julia Gillard (not famed for her grasp of the pronunciation of certain words) once referred to it at “assification.”
https://www.youtube.com/watch?v=IComR2km74Y
She may have been onto something.
The present “science” is becomed a religion.
Narrowminded, arogant and ignorant, a totalitarian mindsett that belongs in religios cults aka The CarboNazis, and if an heredict like me oposes them, they dont argue back nor try to justify their belife, but attacs you with all they have of comon practising I have been upagainst for decades.
Its so corrupted, and what have happend to comon logic, when bollocs are served on a day to day basis, in a never ending stream of pure manure, and this days, coments arent alowed at all, no heretics are alowed to speak into the congregation, against their fire and brimstone tales of the future of our earth, this psycobabbeling is infact what made me react to this shite for years ago, the ability to make our children feel they have comitted a crime for been born.
The level this days is so lousy its downright hillarious.
Like trying to explain the congregation about why the Bigg Bang is pure hoggwash, and I use their own Redshift as my profe against it, since it requires from the result of this shift, and since it has to be an centrall/focal point from where the shift have started, and anywhere they look the universe expands, based upon their messurments of this redshift.
Ok so far.
But how do they then belive our earth is the location of the biggbang, in medival times this was simple geocentrism, and it stil is.
SO either is our earth the point where our universe started from OR the biggbang is a fallacy.
Capice
To me, science is dead.
Dead as snow is a thing of the past in our scientificaly setled world.
Halleluja.
peace